In the search for a cure for diabetes, scientists have stumbled upon the role of liver hepatocytes in pancreatic beta-cell functions. Despite many studies and the development of novel therapeutics, their translation into clinical settings and their effects on disease progression have been modest. One possibility is the complex interplay between islets and other cell types. Studies have unraveled the signaling of islet cells with endothelial cells for angiogenesis. However, the interaction between Human Liver Hepatocytes and islets has become a focal point due to insulin-mediated mechanisms in these cells.
Research on hepatocytes’ effects on islets can enhance the understanding of glucose metabolism and insulin resistance. Their molecular underpinnings can uncover important regulators that can serve as drug targets. Along with drug development it may also delineate disease progression. Research on their interaction has therefore gained significance.
Pancreatic Islets and Liver Hepatocytes
Islets of Langerhans in the pancreas are endocrine cells that release various factors for metabolism. They comprise five distinct subtypes including α-islets (secrete glucagon), β-islets (secrete insulin), γ-islets (secrete pancreatic polypeptide), δ-islets (secrete somatostatin, and ε-islets (secrete ghrelin). Β-islets or Beta-islets represent the highest cell quantity in the pancreas. The function of alpha- and beta-islets is counteracting. Insulin lowers blood glucose while glucagon raises it.
Liver Hepatocytes comprise most of the cell mass and quantity in the liver. They are involved in various functions such as bile synthesis and detoxification. These cells also regulate blood homeostasis by storing glucose as glycogen and synthesizing glucose by gluconeogenesis. Insulin regulates glycogen synthesis and breakdown, thereby shifting hepatic metabolism between glucose storage and production. In addition, insulin also controls lipid and protein metabolism in the liver. It establishes a strong connection between beta-islets and hepatocytes.
Interaction between Beta-islets and Hepatocytes
Several liver pathologies and diabetes coexist, alluding to a connection between both tissues. Type 2 diabetes mellitus can predict the progression of liver disease NAFLD into non-alcoholic steatohepatitis (NASH). Islets secrete insulin and glucagon to alter hepatic metabolism. Furthermore, pancreatic-derived factor (PANDER) also binds to hepatocytes to inhibit the activation of proteins stimulated by insulin. But the cross-talk between them is not unidirectional.
The liver also secretes ANGPTL8 and HGF, which increase beta-cell proliferation and improve glucose-stimulated insulin secretion from beta cells. On the other hand, fetuin A and SeP have contradictory effects on beta cells. Therefore, beta cells govern the metabolism in the liver, whereas the liver regulates beta-cell proliferation. This explains the co-occurrence of pathologies in both tissues.
Islet Transplantation
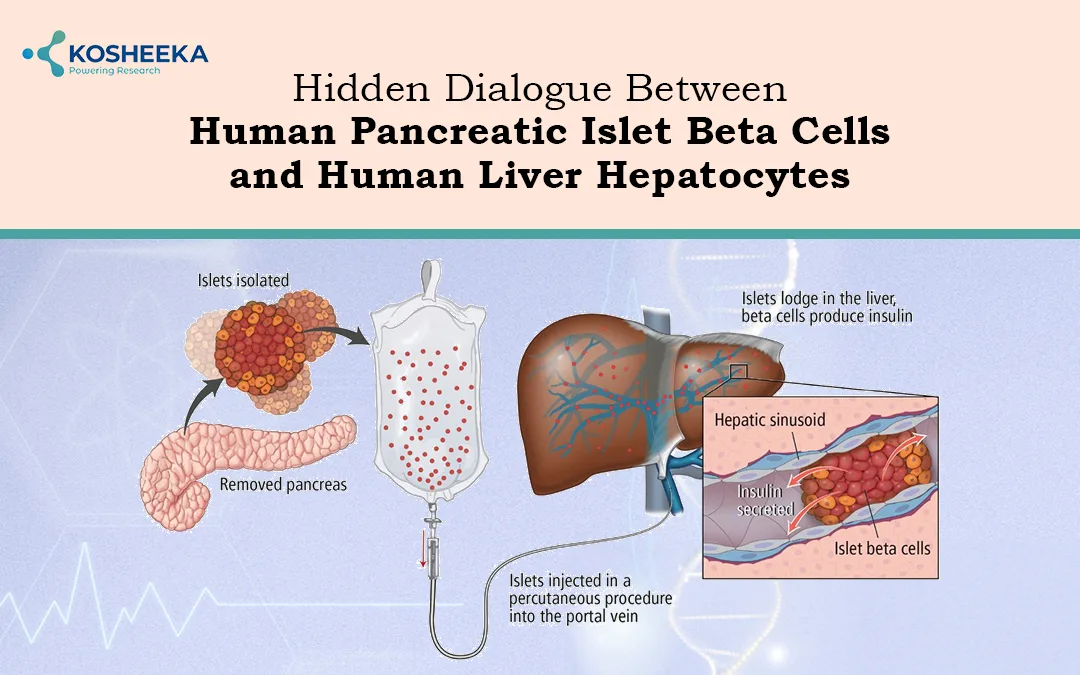
Transplantation of pancreatic islets has been an effective approach for diabetes treatment. The liver is the most common transplantation site that supports the islet cells’ metabolic functions. However, portal vein thrombosis, low islet survival, and suboptimal graft performance impede the success of the transplant. Therefore, a low percentage of patients receiving transplants are able to maintain insulin independence for up to a year. Scientists are exploring other transplantation sites; however, they are associated with instant blood-mediated inflammatory reactions (IBMIRs).
Islet encapsulation has been employed as a strategy to overcome these hurdles. While polymeric encapsulation has yielded positive outcomes in animal models, its success in clinical trials has been limited. Researchers are even exploring a new avenue of encapsulation by other cells. A study published in the Diabetes journal shielded islets with hepatocytes and fibroblasts, demonstrating a reduction in post-transplant islet mortality. Such studies can potentially increase the transplant success rate.
Sources of Beta-Islets
Islet cell transplantation has offered remarkable results in diabetic patients. However, the lack of donor cells is a shortcoming of the process. It has directed the attention of the scientific community towards sources of pancreatic islets, including Stem Cells and hepatocytes. Hepatocytes have drawn considerable interest due to their similar developmental origins to those of the pancreas. They both differentiate from the common endoderm progenitors and share similar expression of genes like GLUT2 and GK. This makes them ideal candidates for reprogramming into pancreatic islet cells.
Reports have shown that they can express transcription factors such as PDX1, Ngn3, MaFA, etc., facilitating their transformation into insulin-producing cells. Both genetic and chemical reprogramming of hepatocytes have been studied. Adenovirus-mediated transfer of genes for these transcription factors individually or in combination have developed insulin-positive cells from hepatocytes. Chemical reprogramming utilized a different set of chemicals for each stage of transdifferentiation (Fig 1).
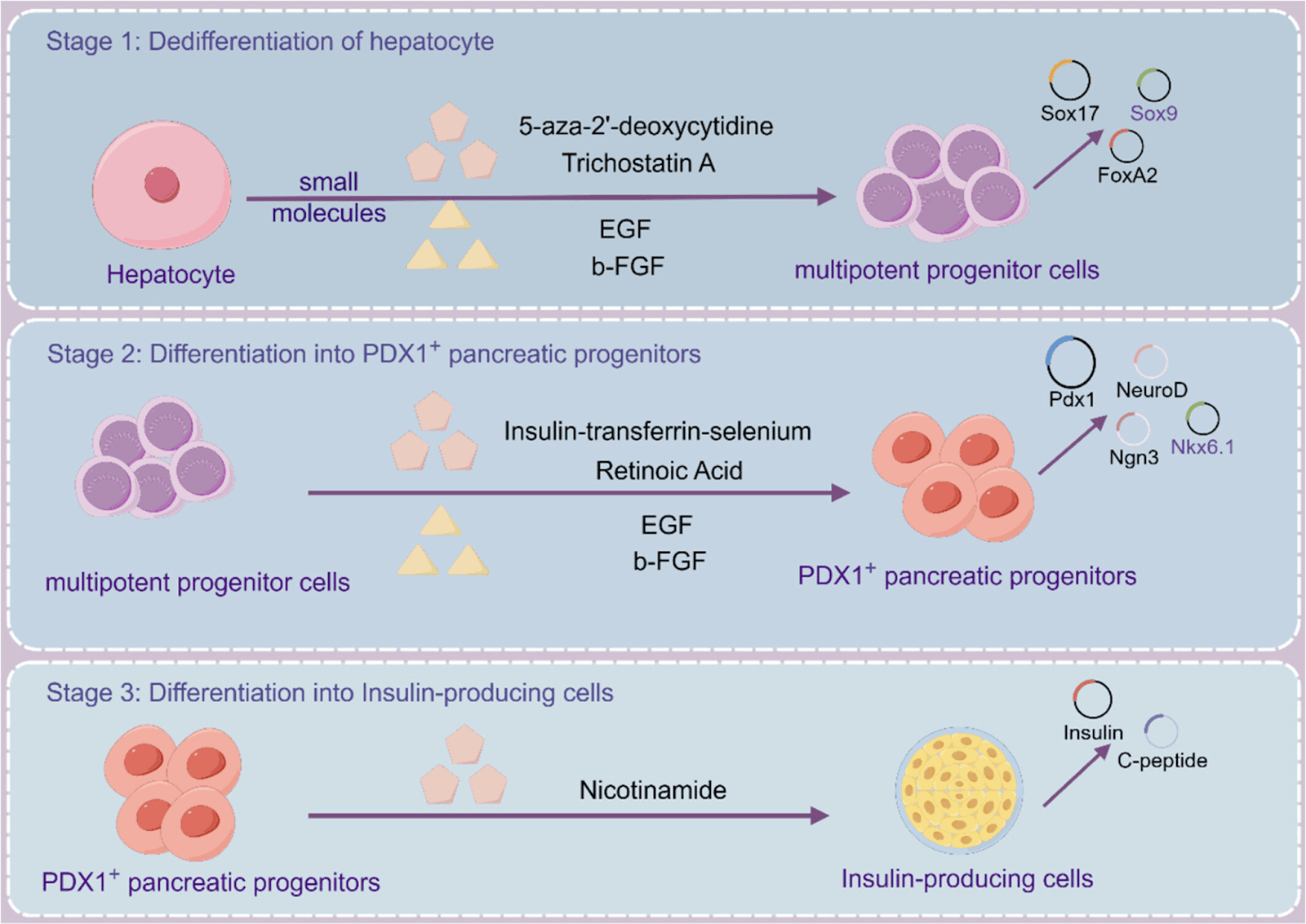
Figure 1. Chemical reprogramming of liver hepatocytes into pancreatic beta-islets. (Source PMID: 38599852)
Islet Beta Cell Regeneration
Replenishing lost islet cells or regulating insulin levels is the primary concern in diabetes treatment. Islet transplantation is a suitable paradigm; however, there has been an inclination toward in vivo islet regeneration. Research on cross-talk between hepatocytes and islet cells has revealed some intriguing results. For instance, serum obtained from a hepatocyte insulin receptor knockout mouse model supported the proliferation of beta cells. Another study identified serpin B1, a liver-secreted factor that increases beta-cell proliferation in this mouse model. Furthermore, liver Igfbp1 stimulates the transdifferentiation of alpha-islets to beta-islets.
A research study also established the role of alpha cell glucagon and the liver FGF21 axis in beta cell regeneration. A research group demonstrated beta cell regeneration by overexpressing hepatocyte genes—the long isoform of the mocs2 enzyme. These studies establish that hepatocytes play an essential role in beta cell quantity. Regeneration via liver factors could prove to be a valuable tactic.
3D Culture
Three-dimensional co-culture models have become popular for demonstrating intercellular interaction. Cells in culture can self-assemble into organoids that self-renew while maintaining characteristics of native tissue. In comparison to 2D culture, they replicate the in vivo spatial organization required for complex physiological processes. The significance of communication between hepatocytes and islet cells has propelled the organoid formation employing both cell types. This 3D coculture of cells on micropores improved the in vivo survival time of the graft while also displaying higher stability and closer junctions.
The 3D co-culture model can also be beneficial in studying the regulatory mechanisms of these cells. Bauer et al. created a two organ-on-chip model (Fig 2). The model spatially distributed both cell types into different compartments and coupled them functionally through a microfluidic channel. It exhibited insulin-based glucose uptake in the liver, proving the robustness of the model.
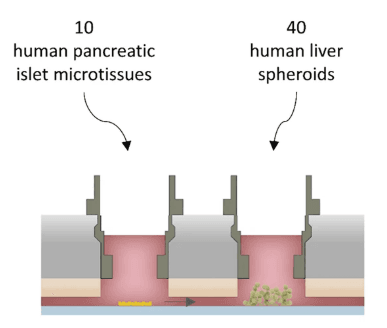
Figure 2. Diagrammatic representation of two organ-on-chip model (Source PMID: 29097671)
Conclusion
Unlike the previous belief that diabetes occurs only in the pancreas, the new observations indicate that it is a multi-organ disease. Therefore, the response to treatment depends on the islets and their interaction with other cells. The bidirectional talk between islets and hepatocytes is essential for nutritional metabolism and beta-cell function. Studies on this interaction have shown beta-cell proliferation through Human Liver Hepatocytes-secreted factors. Hepatocytes differentiation into islet cells and their co-transplantation have overcome challenges such as the shortage of islet cells and in vivo graft survival. Further research can result in the development of a diabetes cure on a holistic level. To promote this research, Kosheeka offers Human Pancreatic Islet Beta Cells and Human Liver Hepatocytes. These cells undergo stringent tests to ensure quality in terms of purity and viability.
Product-Related Queries, Or Partnership Inquiries
FAQ’s
Q- Why is the interaction between pancreatic islets and Human Liver Hepatocytes important in diabetes research?
The cross-talk between these two cells is crucial because it helps regulate glucose metabolism, insulin resistance, and beta-cell proliferation.
Q- What challenges are faced in pancreatic islet transplantation for diabetes treatment?
Although islet transplantation is an effective approach to restore insulin production, challenges such as low islet survival, portal vein thrombosis, and poor graft performance limit long-term success.
Q- Can Human Liver Hepatocytes be used as a source for pancreatic islets?
Yes. Due to their common developmental origin with pancreatic cells, hepatocytes can be reprogrammed into insulin-producing cells. They share similar gene expressions (like GLUT2 and GK) and have strong regenerative capacity.
Q- How are 3D culture models advancing diabetes research?
3D co-culture models allow pancreatic islets and hepatocytes to grow in an environment that mimics real tissues. This setup enhances survival, improves cell-to-cell communication, and provides better insights into metabolic regulation.