The Immuno-Oncology
Featured Products
- Wistar Rat Whole Blood
- Wistar Rat Serum
- Wistar Rat Plasma
- Wistar Rat Liver S9
- Wistar Rat Liver Microsomes
- Wistar Rat Liver Cytosol
- Wistar NK cells
- Wistar Mononuclear cells
- Wistar Mesenchymal stem cells
- Wistar Dermal fibroblasts
- Wistar Dendritic cells
- Villous Mesenchymal Stem Cells
- Umbilical Cord Blood Derived Dendritic Cells
- Swiss Albino Mouse Lung S9
- Swiss Albino Mouse Liver S9
- Swiss Albino Mouse Liver Microsomes
- Swiss Albino Mouse Liver Cytosol
- Swine Skeletal Muscle Fibroblasts
- Swine Primary Bone Osteoblasts
- Swine Primary Bone Osteoblasts
- Swine Pancreatic Islets Cells
- Swine Lung Alveolar Cells
- Swine kidney Fibroblasts
- Swine Hepatocytes
- Swine Dermal Fibroblats
- Swine Cardiomyocytes
- Swine Cardiac Fibroblasts
- Swine Bone Marrow Mononuclear Cells
- Skin Dermal cells
- SD Rat Whole Blood
- SD Rat Serum
- SD Rat Plasma
- SD Rat Liver S9
- SD Rat Liver Microsomes
- SD Rat Liver Cytosol
- SD Rat Intestine S9
- SD Rat Intestine Cytosol
- SD Rat Intestinal Microsomes
- SD NK cells
- SD Muse cells
- SD Mononuclear cells
- SD Mesenchymal stem cells
- SD Dermal fibroblasts
- SD Dendritic cells
- Rhesus Monkey Whole Blood
- Rhesus Monkey Serum
- Rhesus Monkey Plasma
- Rat Schwann Cells Wistar
- Rat Schwann Cells SD
- Rat Schwann Cells Immuno-deficient
- Rat Pulmonary Fibroblasts Wistar
- Rat Pulmonary Fibroblasts SD
- Rat Pulmonary Fibroblasts Immuno-deficient
- Rat Lymphatic Fibroblasts Wistar
- Rat Lymphatic Fibroblasts SD
- Rat Lymphatic Fibroblasts Immuno-deficient
- Rat Hepatocytes Suspension Wistar
- Rat Hepatocytes Suspension SD
- Rat Hepatocytes Suspension Immuno-deficient
- Rat Hepatocytes Plateable-Wistar
- Rat Hepatocytes Plateable-SD
- Rat Hepatocytes Plateable-Immuno-deficient
- Rat Cardiomyocytes Wistar
- Rat Cardiomyocytes SD
- Rat Cardiomyocytes Immuno-deficient
- Rat Cardiac Fibroblasts Wistar
- Rat Cardiac Fibroblasts SD
- Rat Cardiac Fibroblasts Immuno-deficient
- Rat Brain Vascular Pericytes Wistar
- Rat Brain Vascular Pericytes SD
- Rat Brain Vascular Pericytes Immuno-deficient
- Rat Bone Marrow Derived NK Cells Wistar
- Rat Bone Marrow Derived NK Cells Immuno-deficient
- Rat Bone Marrow Derived Muse Cells Wistar
- Rat Bone Marrow Derived Muse Cells SD
- Rat Bone Marrow Derived Muse Cells
- Rat Bone Marrow Derived Mononuclear Cells Wistar
- Rat Bone Marrow Derived Mononuclear Cells Immuno-deficient
- Rat Bone Marrow Derived Mononuclear Cells
- Rat Bone Marrow Derived Mesenchymal Stem Cells Wistar
- Rat Bone Marrow Derived Mesenchymal Stem Cells SD
- Rat Bone Marrow Derived Mesenchymal Stem Cells Immuno Deficient
- Rat Bone Marrow Derived Dendritic Cells Wistar
- Rat Bone Marrow Derived Dendritic Cells SD
- Rat Bone Marrow Derived Dendritic Cells Immuno-deficient
- Primary Hepatocytes Plateable C 57
- Primary Hepatocytes in Suspension CD-1
- Peripheral Blood-Derived Muse Cells
- Pancreatic islets beta cells
- Muse Cells
- Mouse Primary Bone Marrow Derived NK Cells CD1
- Mouse Primary Bone Marrow Derived NK Cells C57
- Mouse Muse cells CD1
- Mouse Muse cells C57
- Mouse Muse cells BalbC
- Mouse Hybrid Liver S9 Fraction Mixed Gender
- Mouse Derived Mesenchymal Stem Cells
- Mouse Derived Dendritic Cells
- Mouse DBA S9 Fraction Mixed Gender
- Mouse DBA Lung S9 Fraction Mixed Gender
- Mouse DBA Liver S9 Fraction Mixed Gender
- Mouse Cytosol Mixed Gender
- Mouse Cardiomyocytes C57
- Mouse Cardiomyocytes BalbC
- Mouse Cardiac Fibroblasts C57
- Mouse Cardiac Fibroblasts BalbC
- Mouse C57 BL/6N Liver S9 Fraction Mixed Gender
- Mouse Brain Vascular Pericytes
- Mesenchymal Stem Cells
- Macaque Monkey blood mononuclear cells
- Lung alveolar cells
- Liver Hepatocytes plateable
- Lewis Rat Whole Blood
- Lewis Rat Serum
- Lewis Rat Plasma
- Kidney Fibroblasts
- Human Whole Blood
- Human Vaginal epithelial cells
- Human Umbilical Cord Blood Derived NK cells
- Human Umbilical Cord Blood Derived Mononuclear cells
- Human Umbilical Cord Blood Derived CD34+ Cells
- Human T Helper Cells
- Human Splenic Fibroblasts
- Human Splenic Endothelial Cells
- Human Skin S9 Fraction Mixed Gender
- Human Skin Derived Microvascular Dermal Endothelial Cells Adult
- Human Skin Derived Epidermal Melanocytes Fetal
- Human Skin Derived Epidermal Melanocytes Adult
- Human Skin Derived Epidermal Keratinocytes Neonatal
- Human Skin Derived Epidermal Keratinocytes Fetal
- Human Skin Derived Epidermal Keratinocytes Adult
- Human Skin Derived Dermal Fibroblasts Fetal
- Human Skin Derived Dermal fibroblasts Adult
- Human Skin Derived Dermal Fibroblasts Adult
- Human Seminal vesicles microvascular endothelial cells
- Human Seminal Vesicles Fibroblasts
- Human Seminal Vesicles Endothelial cells
- Human S9 Fraction Heart
- Human Pulmonary Small Airway Epithelial Cells
- Human Pulmonary Fibroblasts
- Human Pleatable Hepatocytes Pooled
- Human Plateable hepatocytes
- Human Peripheral Blood-Derived NK Cells
- Human Peripheral Blood-Derived Mononuclear Cells
- Human Peripheral Blood-Derived Monocytes
- Human Peripheral Blood-Derived Mesenchymal Stem Cells
- Human Peripheral Blood-Derived Cytotoxic T-Cells
- Human Peripheral Blood Derived Serum
- Human Peripheral Blood Derived Plasma
- Human Pericardial Fibroblasts
- Human Ovarian Surface Epithelial Cells
- Human Ovarian Fibroblasts
- Human Muse cells
- Human Microvascular Endothelial Cells
- Human Mast cells
- Human Mammary Smooth Muscle Cells
- Human Mammary Fibroblasts
- Human Mammary epithelial cells
- Human Lung S9
- Human Lung Microsomes
- Human Lung Cytosol
- Human Liver S9
- Human Liver Microsomes
- Human Liver Cytosol
- Human Kidney Fibroblasts
- Human Islets Beta cells
- Human Islet Beta Cells
- Human Intestine S9
- Human Intestine Microsomes
- Human Intestine Cytosol
- Human Hepatocytes, Plateable
- Human Hepatocytes in Suspension
- Human Eye Derived Primary Retinocytes
- Human Eye Derived Limbal Fibroblasts
- Human Extra Embryonic Fetal Tissues Muse cells
- Human Extra Embryonic Fetal Tissues Derived CD34 Positive Cells
- Human Extra Embryonic Fetal Tissues Dendritic Cells
- Human Endometrial Epithelial Cells
- Human Cytotoxic T Cells
- Human Cord Blood Derived Serum
- Human cord blood derived Plasma
- Human Cardiomyocytes
- Human Cardiac Fibroblasts
- Human Bronchial Fibroblasts
- Human Bone Marrow-Derived NK Cells
- Human Bone Marrow-Derived Mononuclear cells
- Human Bone Marrow-Derived Mesenchymal Stem Cells
- Human Bone Marrow-Derived Dendritic cells
- Human Bone Marrow-Derived CD 34 positive cells
- Human Bone Marrow Blood Derived Serum
- Human bone marrow blood derived Plasma
- Human Aortic Smooth Muscle Cells
- Human Aortic Endothelial Cells
- Human Adipose Tissue-Derived Stromal Vascular Fraction
- Human Adipose Tissue-Derived Preadipocytes
- Human Adipose Tissue derived Mesenchymal Stem cells
- Horse peripheral blood mononuclear cells
- Horse mesenchymal stem cells-adipose tissue
- Hepatic Stellate Cells
- Golden Syrian Hamster Serum
- Golden Syrian Hamster Plasma
- Gingival Fibroblasts
- Endothelial cells
- Dog mesenchymal stem cells adipose tissue
- Dog hepatocytes plateable
- Dog blood mononuclear cells
- Dental Pulp Mesenchymal Stem Cells
- Dendritic cells
- Cynomolgus Monkey Serum
- Cynomolgus Monkey Plasma
- Cynomolgus Monkey blood mononuclear cells
- Cynomolgus cryopreserved hepatocytes, plateable
- Cynomolgus cryopreserved hepatocytes, plateable
- CD-1 Schwann cells
- CD-1 Pulmonary fibroblasts
- CD-1 NK cells
- CD-1 Muse cells
- CD-1 Mouse Whole Blood
- CD-1 Mouse Serum
- CD-1 Mouse Plasma
- CD-1 Mouse Lung S9
- CD-1 Mouse Lung Microsomes
- CD-1 Mouse Lung Cytosol
- CD-1 Mouse Liver S9
- CD-1 Mouse Liver Microsomes
- CD-1 Mouse Liver Cytosol
- CD-1 Mouse Intestine S9
- CD-1 Mouse Intestine Microsomes
- CD-1 Mouse Intestine Cytosol
- CD-1 Mononuclear cells
- CD-1 Mesenchymal stem cells
- CD-1 Hepatocytes plateable
- CD-1 Dermal Fibroblast
- CD-1 Dendritic cells
- CD-1 Cardiomyocytes
- CD-1 Cardiac fibroblasts
- CD-1 Brain vascular pericytes
- Cardiomyocytes
- Cardiac fibroblasts
- C57 Schwann cells
- C57 Pulmonary fibroblasts
- C57 NK cells
- C57 Muse cells
- C57 Mouse Whole Blood
- C57 Mouse Skin S9
- C57 Mouse Skin Microsomes
- C57 Mouse Skin Cytosol
- C57 Mouse Serum
- C57 Mouse Plasma
- C57 Mouse Lung S9
- C57 Mouse Lung Microsomes
- C57 Mouse Lung Cytosol
- C57 Mouse Liver S9
- C57 Mouse Liver Microsomes
- C57 Mouse Liver Cytosol
- C57 Mouse Intestine S9
- C57 Mouse Intestine Microsomes
- C57 Mouse Intestine Cytosol
- C57 Mouse Heart S9
- C57 Mouse Heart Microsomes
- C57 Mouse Heart Cytosol
- C57 Mononuclear cells
- C57 Mesenchymal stem cells
- C57 Hepatocytes Suspension
- C57 Dendritic cells
- C57 Cardiomyocytes
- C57 Cardiac fibroblasts
- C57 Brain vascular pericytes
- Brown Norway Rat Whole Blood
- Brown Norway Rat Serum
- Brown Norway Rat Plasma
- Beagle Whole Blood
- Beagle Serum
- Beagle Plasma
- Beagle Dog hepatocytes cryopreserved, plateable
- BalbC Schwann cells
- BalbC Pulmonary fibroblasts
- BalbC NK cells
- BalbC Muse cells
- BALBC Mouse Whole Blood
- BALBC Mouse Serum
- BALBC Mouse Plasma
- BalbC Mononuclear cells
- BalbC Mesenchymal stem cells
- BalbC Hepatocytes Suspension
- BalbC Hepatocytes plateable
- BalbC Dermal Fibroblasts
- BalbC Dendritic cells
- BalbC Cardiomyocytes
- BalbC Cardiac fibroblasts
- BalbC Brain vascular pericytes
- BALB/c Mouse Skin S9
- BALB/c Mouse Skin Microsomes
- BALB/c Mouse Skin Cytosol
- BALB/c Mouse Lung Cytosol
- BALB/C Mouse Liver S9
- BALB/c Mouse Liver Microsomes
- BALB/c Mouse Liver Cytosol
- BALB/c Mouse Intestine S9
- BALB/c Mouse Intestine Microsomes
- BALB/c Mouse Intestine Cytosol
- BALB/c Mouse Heart S9
- BALB/c Mouse Heart Microsomes
- BALB/c Mouse Heart Cytosol
- Amniotic Epithelial cells
Drop your Query
With recent research, cancer immunotherapy has notably been proven to be effective in eradicating or minimizing the spread of invading primary as well as metastatic tumors. In principle, cancer immunotherapeutics are based on the stimulation of the body’s immune cells against the elimination of cancerous cells; and are accomplished with the help of several methods including but not limited to cytokines, vaccines, adoptive cellular therapy, and oncolytic viruses.
Apart from cancers, autoimmune systems have also gained special interest due to the ability of normal immune cells of the body to attack its cells. Accordingly, multiple projects are going on across the world to facilitate different studies including toxicity analysis, graft rejection studies, and studies related to inflammation, allergies, and drug development.
Kosheeka has a range of primary immune cells including NK cells, monocytes, macrophages, and dendritic cells. You just have to design and test treatments as part of your research and development.
In the current age of immunotherapeutics, which is targeting tumor-producing microenvironment (SMEs), the need for immune models prepared by a range of primary immune cells, and further integration of human immune cells are in growing demand. Despite recent advancements in creating xenografts or humanized mice, the drug development of cancer immunotherapeutics relies more on syngeneic animal models due to their intact immune systems (Oslon et.al.; 2018).
Contrary to this, engineered human cells or primary human cells offer similar in vivo complexity, while fully retaining physical parameters as well as the cellular composition of a particular organ. This encouraged many researchers to use primary cellular systems to recapitulate the physiological state of the cells within a body and study underlying physical as well as chemical signaling patterns of a particular tumor microenvironment (TME) (Hue et.al.; 2011).
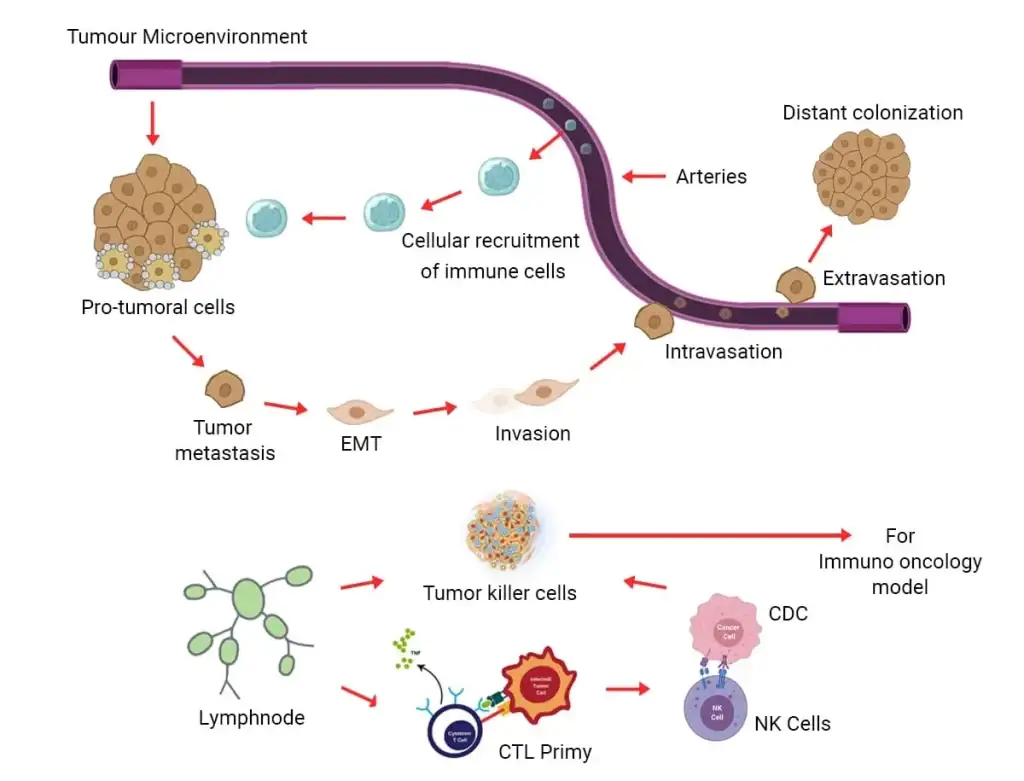
In a plethora of upcoming studies interaction between cancer cells and the immune system can be a decision-maker in controlling tumor outgrowth or tumor metastasis. Studies have also indicated tumor-promoting inflammation as well as escaping the process of immune-mediated destructions to be the bonafide hallmarks of growing cancer. These experimental models can turn out to be powerful tools for validating results from a clinical specimen. Chimeric antigen receptor T-cell therapy (CAR T) is a key example, to re-engineer a patient’s T-cells to fight their cancer. Monoclonal antibodies (mAbs) and immune checkpoint inhibitors are further examples of immunotherapy.
Kosheeka offers a comprehensive portfolio of reliable primary cells, media, reagents, and kits for immune-oncology research. The HLA-typed human primary cells from specific organs enable the testing of new immunotherapies for off-target effects, with the potential to request certain donor types.
The Kosheeka Primary Cancer Culture System (PCCS) allows selective isolation of long-term primary cultures of malignant cells from tumor samples, allowing researchers to deplete benign cells from culture and selectively maintain malignant cells. With the growing importance of immunotherapies, Kosheeka is ready with its range of products required for experimental models, such as:
- Cytotoxic T cells
- T Helper 1 Cells
- T Helper 2 Cells
- Regulatory T Cells
- B Cells
- Macrophage Cells
- Dendritic Cells
- Natural Killer Cells (NK Cells)
- Muse Cells
- Customized primary cancer cells
- Optimized cell culture growth media
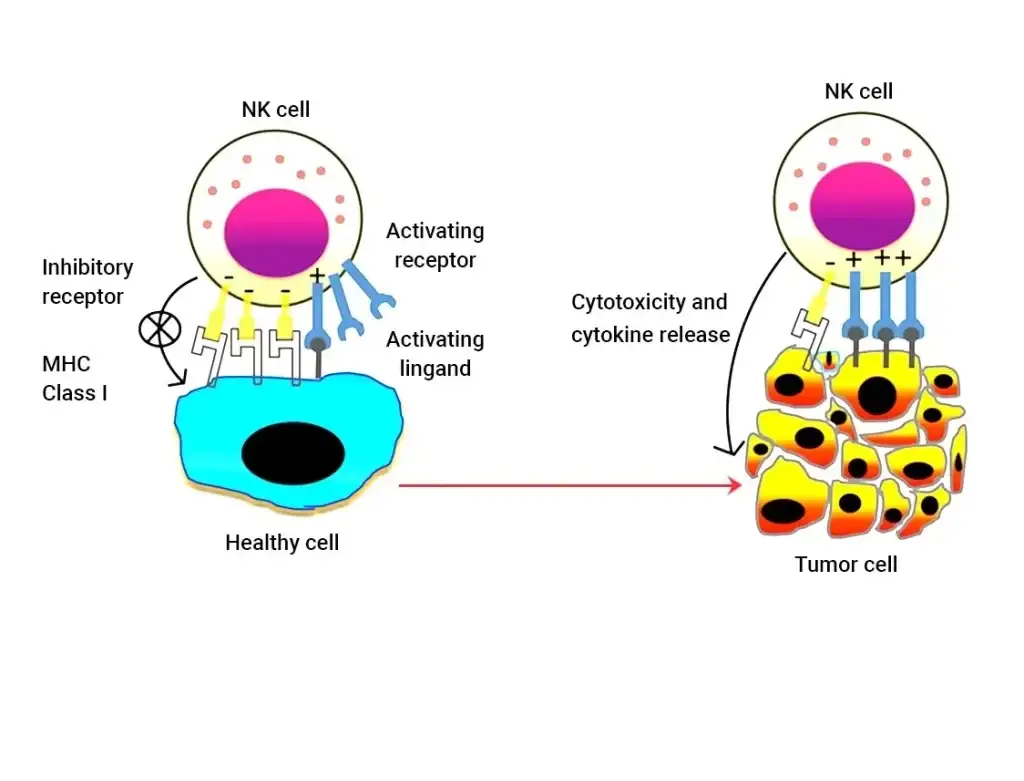
NK cells
These are the predominant cells present in the innate lymphocyte subsets and are responsible for mediating anti-tumor response or anti-viral response; depending upon the inflammatory profile of the body. With this property, they represent a promising tool for clinical utilization in the case of clinical oncology. Importantly, some recent studies on the pandemic have also revealed NK cells’ ability to mediate anti-viral effector functions with the help of a set of evasion mechanisms.
NK cells as anti-viral models
Different studies are going to identify the relevance of NK cells in mediating anti-viral immune response, and how this important property can be utilized against the spread of virus infection in the form of immunotherapy. Importantly, researchers can find a clue that with the help of memory NK cells, can be adoptively transferred to another naïve host to initiate an appropriate immune response against developing pathogenic entry.
NK Cells mediated anti-tumor mechanism
While NK cells isolated from lymphocytes, represented natural cytotoxicity in certain tumor cells, even in the absence of preimmunization; such as CD56dim NK cells, making up the majority of circulating tumor cells. Studies have also confirmed that these high levels of tumor-infiltrating NK cells are responsible for positive prognostic tumor markers. However, it is very crucial to understand how NK cells are recognized by inhibitory and activating receptor complexes. Hence, three recognition models have been studied exclusively, such as ‘missing self’, ‘non-self’, and ‘stress-induced self’.
Interestingly, there are also various studies planned to understand how tumor cell receptors are planning to escape the surveillance of NK cells, which may be either by losing the expression of adhesion molecules, or upregulation of certain class I MHC complex. Given the importance of NK cells produced clinically, various clinical studies have been designed to understand their therapeutic importance.
- Autologous NK cells were used on patients with metastatic RCC, malignant glioma, and breast cancer. The trial confirmed the limited activity of autologous NK cells (Farag SS, et.al; 2004).
- Allogeneic NK cells were used on patients with metastatic melanoma, renal cell carcinoma, and Hodgkin’s disease; and were found to be safe with minimal toxicity (Hayes RL. et.al; 1995).
- Systemic administration was facilitated using allogeneic NK cells on a patient with Hodgkin’s lymphoma; which was found to have expressed higher cytotoxicity to antibody-coated targeted cells (Berzofsky JA. et.al; 2001).
- Another trial was completed with genetically modified NK cells; which found successful anti-tumor effects along with limited specificity of NK cells (Escudier B, et.al; 1994).
Dendritic Cells
They are very important antigen-presenting cells with a unique capacity to activate naïve T Cells. However, their central role in regulating adaptive immune response is yet to be fully appreciated. In the era of lymphocytes, where rulers of immunotherapies like NK cells and dendritic cells (DC) were known but ignored; some researchers’ relentless pursuit to know their functions has understood their potential to translate into clinical research. Dendritic cells are professional antigen-presenting cells and are located throughout the body as avengers capturing invading pathogens, with the help of multiple triggers like toll-like receptors, and phagosomes.
Application of DCs in immune regulation
The genetic models strongly supported the supreme capacity of DCs in T cell priming. Some of the preclinical models have confirmed the idea that DCreg can induce immunogenic tolerance and reverse disease phenotype through immunogenic regulation in models of allergies, asthma, and autoimmune disorders.
Interestingly, several clinical trials have pushed the idea of using DCreg in clinical applications. These models were prepared to assess hypersensitivity reactions, detect interleukin responses, etc (Gordon JR, et.al; 2005). The present in vitro data further suggest that naturally occurring dendritic cells can be a more potent and reasonable alternative to monocyte-derived dendritic cells, considering their availability and clinical potency. The same is in demand by many pharmaceutical companies for the preparation of DC vaccinations.
Thus, the range of products that are being developed at Kosheeka are:
- Monocyte-derived dendritic cells
- Naturally circulating dendritic cells
- Myeloid Dendritic cells
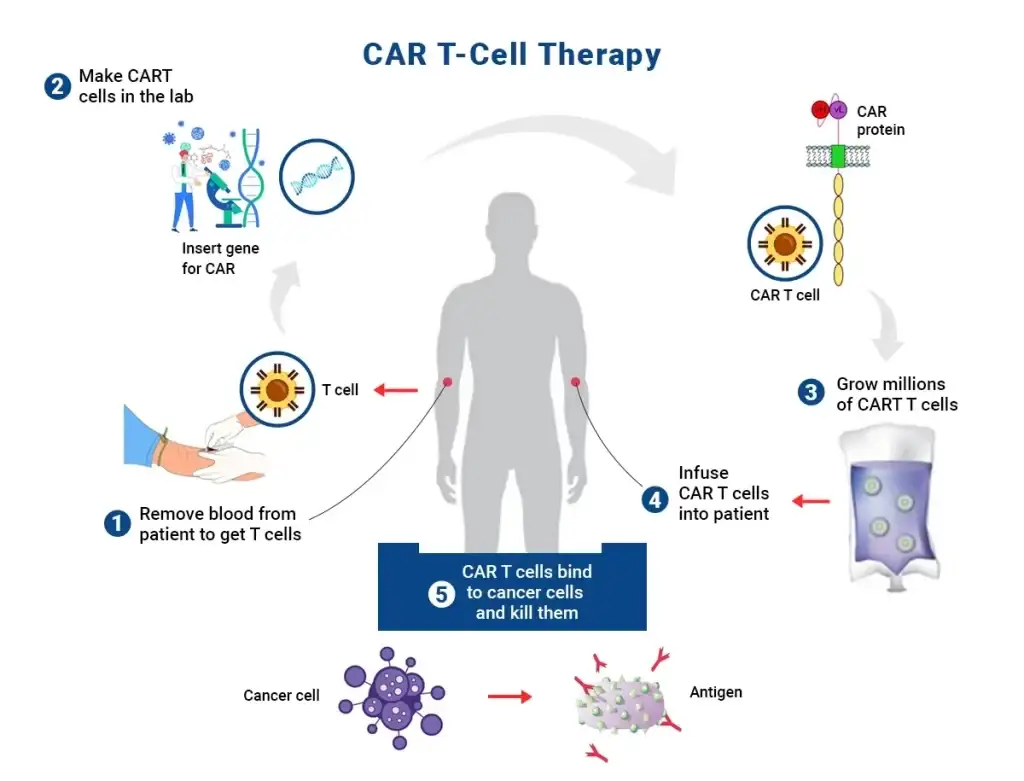
CAR T Cells
The advent of Chimeric Antigen T cell therapy (CAR T) has challenged the traditional foundation of cancer therapeutics involving surgery, chemotherapy as well as radiation treatment. Although these continue to be critical mainstay treatments, with a growing understanding of cellular biology and genetics there is a huge transformation in the field of medical oncology.
CAT-T Cell Therapy: A “Living Drug”
How about the idea of giving patients a living drug that can locate the growing tumor, kill it on the spot, and also eradicate the cells that are infected with pathogenic multiplication? However, researchers are still exploring various side effects that were earlier thought to be associated with therapeutic applications of engineered CAR T cells. Importantly, preclinical mouse/rat models have mostly failed to predict these complications in humans, as they were primarily designed only for testing toxicity at the time of initial observation in patients.
Preclinical models: Cytokine-releasing syndrome (CRS) and neurotoxicity
Some studies have indicated that one of the most common and potentially dangerous side effects of CD19 CAR T cell therapy is CRS and neurotoxicity. As per the American Society of Transplantation and Cellular Therapy (ASTCT), the CRS and its induced neurotoxicity are described as an immune effector cell-associated supraphysiological response following immunotherapies like CAR T cell therapy. Some of the commonly reported symptoms are activation of endogenous T cells, causing fever, hypotension, capillary leak, and organ dysfunction.
CRS is also commonly reported in patients with multiple myeloma post-CAR T cellular therapy. Some of the commonly reported manifestations of neurotoxicity, post-CAR T include confusion, language disturbance, deficits in fine motor skills, encephalopathy, dysphasia, aphasia, cerebral edema with coma, and death. For that matter, across various corners of the world, CAR T cells are evaluated for safety and feasibility with the help of the identification of certain biomarkers. Accordingly, special interests have been developed to study in vitro models created by coculturing with other monolayers of cells; to test the specificity and efficacy of CAR T cells. More recently, other cells like macrophages have also been found to be co-cultured with CART cells, which facilitated mechanistic insights into CRS.
Models for GVHD and rejection
Allogeneic mouse models have been studied for GVHD, post CART therapy; and the model demonstrated limited or no risk due to cumulative signaling initiation pattern by exogenous CAR and endogenous alloreactive TCR, causing possible deletion of transferred T cells, facilitating their functional loss. Alternatively, some studies also employed allogeneic CART cells isolated from peripheral blood mononuclear cells and coculturing them with other macrophages to study the pattern of GVHD and rejection.
Many more such models are being studied worldwide. Kosheeka will also soon be entering the world of immunotherapeutics by offering a range of naturally presenting and genetically engineered CAR T cells.
- Gordon JR, Li F, Nayyar A, Xiang J, Zhang X, et al. CD8 alpha+, but not CD8 alpha-, dendritic cells tolerize Th2 responses via contact-dependent and -independent mechanisms, and reverse airway hyperresponsiveness, Th2, and eosinophil responses in a mouse model of asthma. J Immunol (2005) 175(3):1516–22. doi: 10.4049/jimmunol.175.3.1516
- Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W, et al. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol (2014) 5:7. doi: 10.3389/fimmu.2014.00007
- Escudier B, Farace F, Angevin E, Charpentier F, Nitenberg G, Triebel F, et al. Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL2. Eur J Cancer A. 1994;30A:1078–1083.
- Farag SS, Caligiuri MA. Cytokine modulation of the innate immune system in the treatment of leukemia and lymphoma. Adv Pharmacol. 2004;51:295–318.
- Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840–852.
- Huh D., Hamilton G.A., Ingber D.E. From 3d cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754.
- Parihar R, Nadella P, Lewis A, Jensen R, de Hoff C, Dierksheide JE, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon-gamma production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037.
- Olson B., Li Y., Lin Y., Liu E.T., Patnaik A. Mouse models for cancer immunotherapy research. Cancer Discov. 2018;8:1358–1365.
- Rosenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am. 2000. pp. S2–S7.
