Primary hepatocytes are the closest in vitro model to the liver. They constitute 80% of the hepatic volume. In vitro research on these cells have substantially enhanced our knowledge of liver diseases and function. It has significantly contributed to drug discovery. Hepatocytes are also the preferred in vitro tools for high-throughput screening of drugs for toxicity. The researchers have particularly dived into the regenerative capabilities of the organ with hepatocyte culture. They have ventured into cell-based therapies for the liver to address the rising prevalence of chronic liver diseases.
Human Liver Hepatocytes
The liver parenchyma comprises two epithelial cells –hepatocytes and cholangiocytes. Along with them, the liver also contains stellate cells, endothelial cells, Kupffer cells, and smooth muscle cells. Human Liver Hepatocytes are binucleated polygonal cells arranged as cords and separated by blood-filled spaces. They perform diverse primary functions such as carbohydrate metabolism, albumin secretion, bile synthesis, nutrient storage, cholesterol synthesis, and detoxification. These cells are packed with organelles, particularly mitochondria and endoplasmic reticulum, that assist in their high metabolic activity. The surface of hepatocytes is either – sinusoidal (adjacent to blood vessels), canalicular (apposed to canaliculi for bile excretion), or intercellular (adjacent to other hepatocytes). Hepatocytes display functional diversity with respect to their location and association with other cells (Fig 1).
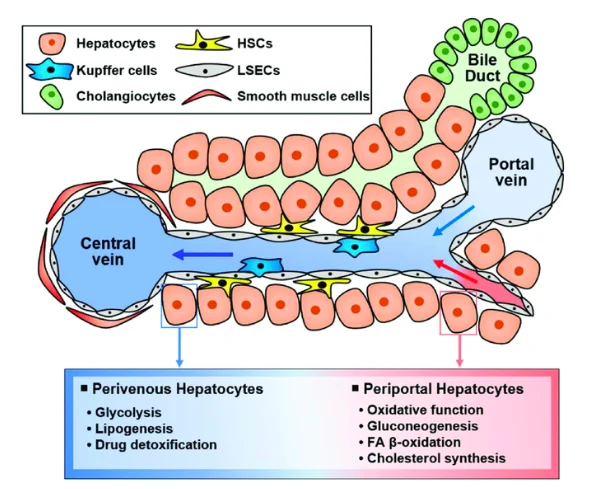
Figure 1. Diverse functions of hepatocytes according to their location. (Image courtesy: PMID: 37635938)
Human Liver Hepatocyte Isolation
Cell isolation from tissue usually employs mechanical or enzymatic dissociation. However, the mechanical method of cell harvesting from hepatic tissue can result in cellular damage and subsequent lower yields. Therefore, enzymatic method using collagenase have gained preference. The technique entails either perfusing the intact or whole liver in the enzyme solution or digesting a hepatic tissue slice in the solution.
Seglen introduced a two-step process involving the enzymatic digestion method. The first step followed the perfusion of the liver with warm (37˚C) Ca2+-free buffer to lose the desmosome junctions holding cells together. The second step employed perfusion with a collagenase solution containing Ca2+ to break the contact of cells with the extracellular matrix (ECM). Afterwards, low-speed centrifugation resulted in a high yield of the hepatic cells. Density gradient centrifugation or magnetic-activated cell sorting further separates a pure population of hepatocytes devoid of other hepatic cells.
Human Liver Hepatocytes Culture
The hepatocyte culture has been challenging due to the short life span and cell differentiation into other lineages. Therefore, attempts have been made to imitate their in vivo niche. For instance, hepatocyte culture on collagen-coated culture dishes, which mimics the hepatic ECM. Cells were able to secrete urea and albumin with minimal cytochrome P450 activity in these conditions, but only for a week.
Therefore, Dunn et al. proposed a new approach in 1989 for hepatocyte culture- the sandwich model. The model added a second layer of collagen over the hepatocytes plated on a collagen-coated culture dish. Now these cells retained their morphology for 42 days in culture, along with gradual stabilization in their secretory function. The co-culture model of hepatocytes with non-parenchymal cells, such as microvascular cells or fibroblasts, integrating cell-cell interactions, has also been effective. Development of such models can enhance the in vitro hepatocyte survival, facilitating their culture.
Applications in Drug Development
Evaluation of novel drugs for absorption, digestion, metabolism, excretion, and toxicity (ADMET) properties is mandatory. Liver is the key organ involved in xenobiotic metabolism; therefore, primary hepatocytes are suitable for analysis of drug metabolism and toxicity.
Drug Metabolism
Hepatocytes comprise Phase I (responsible for oxidation, reduction, and hydrolysis) and Phase II (responsible for conjugation) enzymes. Cytochrome P450 is a key phase I enzyme. Through these enzymes, investigators can study:
- Drug uptake
- Drug kinetics
- Hepatic clearance
- Metabolites of drugs produced by hepatic tissue
- Drug-drug interactions
These studies facilitate the prediction of the safety of the drugs before proceeding to animal studies. It substantially reduces the cost, labor, and time invested in preclinical research.
Drug Toxicity
Drug-induced hepatotoxicity can occur due to mitochondrial damage, metabolic inhibition, and disrupted hepatic functions. The changes in cell cycle and DNA damage in primary hepatocytes also aid in detecting potential carcinogens. Hepatocytes exhibit the tissue functions and thus are a reliable model for assessing Drug Cytotoxicity to hepatic cells.
Applications in Transplantation
The growing incidence of hepatic damage or failure has increased the demand for transplant options. The shortage of donors for transplants leads to long waiting times and a higher mortality risk. Therefore, scientists have been exploring alternatives using hepatic cells.
Hepatocyte Transplantation
Chronic or acute liver failure and genetic disorders necessitate the transplantation of healthy cells. It replaces damaged tissue cells with healthy counterparts to improve liver structure and function. Transplants of primary hepatocytes seem a viable alternative. Early research in cases of liver failure has shown considerable improvements after the transplant. Animal studies have demonstrated prolonged survival and improved tissue function following transplant. However, the transplant procedure requires the optimization of cell dosage, the administration route, and the need for repetitive cell infusion.
Bioartificial Liver
It is an external device containing hepatocytes. It is typically used for short periods of time to support the hepatic functions until a donor for a liver transplant is available. The working principle involves circulation of a patient’s blood through the device. The cells within the device process the blood before returning it to the patient’s bloodstream.
Bioprinting
One of the revolutionary ideas for transplant therapies is to grow a whole liver in a lab. With the rising demand for orthotopic liver transplants, scientists have moved in the direction of three-dimensional bioprinting of the hepatic tissue. These techniques – 3D culture and bioprinting have become popular in research and therapy development. Therefore, bioprinting of the liver for transplant has been attempted. These bioprinted tissues have extended the survival period, as evident in the animal studies. This technology can overcome the hurdles of donor shortage. However, different aspects of the technique require streamlining before clinical translation.
Applications in Research
Virology
The liver is susceptible to viral infection, particularly HCV and HBV. Therefore, hepatocytes serve as a suitable model to study the underlying processes, such as attachment and entry of viruses. This can drive the generation of new drug candidates and vaccines.
Regeneration
The regeneration ability of the liver has always fascinated scientists. Their signaling mechanisms and the cells responsible have been the subject of research. Recent studies have suggested that hepatocytes participate in the process. They proliferate and replenish the lost cells. Additionally, their reprogramming into hepatic progenitor cells contributes to hepatic regeneration.
Disease Modeling
Chronic liver diseases, such as fatty liver, non-alcoholic fatty liver, Wilson’s disease, viral infections, and autoimmune hepatitis, cause a considerable number of deaths every year. Understanding the disease pathways is essential to developing effective treatment modalities. Primary human liver hepatocytes are the appropriate candidates for conducting research on the disease pathogenesis. Research on hepatocyte-based organoids has empowered the study design by providing a more accurate model for analysing drug absorption and distribution similar to that of in vivo tissue.
Personalized Medicine
Individual variation in physiology and genetic traits can result in different responses to drug treatments. Screening drugs on patient-derived primary human hepatocytes can offer a valuable platform for delineating effects of the drug and individual’s response to the treatment. It can assist in tailoring the treatment plan such as drug dosage, drug selection, etc. accordingly.
Lipid Metabolism
Non-alcoholic fatty liver disease (NAFLD) begins with abnormal lipid accumulation in the tissue. It often leads to inflammation and damage to the liver. Hepatocyte culture can drive the exploration of lipid metabolism in NAFLD and its progression to hepatic steatosis. It holds immense potential to develop therapeutic interventions.
Glucose metabolism
Diabetes has become frequent nowadays, urging scientists to search glucose metabolism pathways.The liver controls various glucose metabolism pathways such as gluconeogenesis, glycolysis, glycogenesis, etc. Therefore, hepatocyte research can identify new targets for treatments.
Bile Acid Synthesis
Bile formation is essential for digestion. Its secretion requires several transporters on the cell surface. In vitro culture of human primary liver hepatocytes can reveal the proteins behind bile synthesis and secretion, the type and subcellular location of transporter proteins, and the effects of drugs on bile secretion.
Culture
Hepatocytes have a limited life span in the in vitro culture. It restricts their use in culture, demanding repetitive isolation and precise experimental planning. Scientists have been attempting to comprehend and enhance their proliferation, such as generating spheroids and identifying growth factors, along with their effects on the cell cycle and the responsible signaling pathways. It can boost the application of primary human hepatocytes for in vitro studies. The increase in survival and proliferation will also improve the availability of cells for various assays.
Liver-on-a-chip
The limitation of two-dimensional culture can be overcome by three-dimensional (3D) culture. Liver-on-chip facilitates 3D hepatocyte culture in a microfluidic device. It promotes the alignment of cells in a specific construct, such as the hepatic cord or lobes. This 3D Cell Culture is a much better representation of in vivo organs and can divulge more key information about the physiological functions of tissue.
Product-Related Queries, Or Partnership Inquiries
The Concluding Statement
Primary human hepatocytes are indispensable tools for preclinical drug screening. Their utilization in transplantation, disease modeling, and regeneration studies has opened new avenues in research and therapy. The in vitro culture of these cells faces some challenges. Since the optimization of their isolation, hepatocyte culture has made long strides. The advent of 3D culture technology might soon ease the hepatocyte culture, by improving their life span. Kosheeka provides human primary liver hepatocytes for your research. Our team rigorously tests each batch to ensure viability, purity, and sterility to expedite your research.
FAQ’s
Q- How are hepatocytes isolated from the liver?
The isolation procedure uses a two-step technique. In the first step, a calcium-free buffer is perfused through the liver to loosen the junctions between cells (these junctions are calcium-dependent). In the next step, the calcium-containing collagenase enzyme is perfused to dissociate the cells from the hepatic extracellular matrix. Thereafter, cells are separated by low-speed centrifugation and further processed by density-gradient centrifugation or magnetic-activated cell sorting to collect a pure population of hepatocytes.
Q- How are hepatocytes cultured in an in vitro laboratory?
Hepatocytes are cultured on collagen-coated culture dishes in an in vitro laboratory. Additionally, a sandwich model is employed. In this model, a hepatocyte layer is grown on a collagen-coated dish, followed by the addition of a collagen layer above the hepatocyte layer.
Q- How are hepatocytes used for drug development?
The liver is the primary organ for drug metabolism. The Phase I and II enzymes involved in the metabolism are present in hepatocytes. Therefore, instead of the whole organ, hepatocytes can be used in vitro to screen drug metabolism and the toxic metabolites that might form in the process. Due to the in vitro culture, high-throughput screening of drugs is also possible. These studies can predict the safety of drugs.
Q- What are the applications of hepatocytes in liver transplants?
Obtaining a suitable donor for a liver transplant is challenging. However, transplants with hepatocytes or printing a whole organ with these cells have potential applications in transplant procedures. Bioartificial livers have also been created with hepatocytes to support liver functions until the transplant procedure.