Mast cells serve as a first line of defence against allergens and parasitic infections. These are innate immune cells that develop from bone marrow hematopoietic stem cells. They also regulate tissue repair and angiogenesis. Their longer lifespan and exposure to diverse stimuli are believed to grant uniqueness to each cell. However, studies have also revealed their association with several disorders, notably expanding their research. One of the key research areas is cardiovascular disorders. The prevalence of cardiac disease and the resulting mortality have been rising in the last few years. The rising research in this area has drawn interest towards mast cells and their contribution to disease pathology.
Mast Cells
Mast cells reside in virtually every vascularized tissue except the brain and retina. They are strategically present at the interface of tissue and environment, such as blood vessels, follicles, glands, etc., with a higher chance of pathogen entry. The progenitors originating in bone marrow travel to tissues to differentiate into Mast Cells.
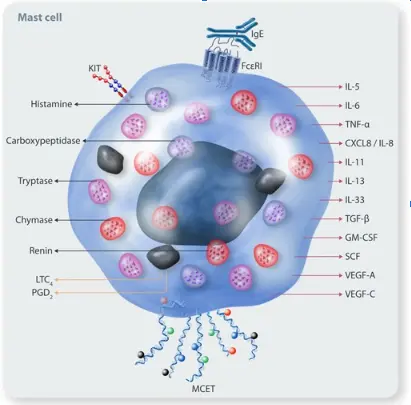
Their activation depends on their IgE receptors- high-affinity FcεRI and low-affinity FcεRIII. IgE binds and cross-links its receptors on mast cells to stimulate degranulation. Additionally, complement factors can also activate them. Another unique receptor is Mas-related-G-protein receptor-2 (MRGPRX2), which responds to stimuli such as snake venom, substance P, antibiotics, etc. Numerous such receptors, including toll-like and cytokine receptors, facilitate cell activation by diverse stimuli (Fig 1). Therefore, these cells are considered the sentinels that respond to alterations in the microenvironment.
Figure 1. Mast Cell Receptors and Mediators (Image Courtesy: PMID:38630620)
Mast Cell Culture
The sources of mast cells are either tissues or progenitors. Tissue derivation involves the extraction of a tissue sample, its enzymatic digestion, and magnetic-activated cell sorting (MACS) to obtain a pure population. Lower cell yield from tissue has promoted the differentiation method. This method employs CD34+ progenitors from bone marrow or peripheral blood. By adding stem cell factor (SCF), IL3, and IL6, the method differentiates progenitors into mast cells. It takes about 6-7 weeks to complete this process. These cells usually do not persist in culture for very long. They comprise a lot of activating and inhibitory receptors on their surface. Among them, c-kit is the key receptor responsible for cell proliferation and development. Therefore, the addition of IL3, SCF, and IL4 in the medium inhibits cell apoptosis and encourages proliferation.
Cell Characterization
Mast cells are oval or irregularly shaped with a single central nucleus. Their dense granules containing inflammatory mediators are visible in the light microscope. Electron microscopy reveals deep plasma membrane invaginations and small pseudopods extending out of the membrane. In the degranulation stage, channels in the membranes become prominent.
The standard surface markers include c-kit (CD117) and FcεRI. Additionally, functional assays can measure mast cell activity. They generally include incubation with IgE and antigen to trigger degranulation. The measurement of inflammatory mediators such as heparin, IL8, etc., substantiates the cell functional activity. Furthermore, toluidine blue staining also aids in characterization. Acidic heparin in these cells turns alkaline toluidine red-violet color—a phenomenon known as metachromasia. After degranulation, toluidine blue stains these cells pink.
Mast Cell Lines
The definite lifespan of mast cells has prompted the application of their cell lines with indefinite proliferation. Since 1988, many cell lines have been extracted from patients with mast cell-specific disorders. Some common examples include-
- LAD2 (Laboratory of Allergic Diseases 2) is an SCF-dependent human cell line extracted from a patient suffering from mastocytosis. LAD2 lines grow relatively slowly and produce fewer cytokines compared to primary cells from human progenitors.
- HMC1 (Human Mast Cell Leukemia-1) has mutations activating the c-Kit receptor, leading to cytokine production but limited degranulation capacity.
Other human cell lines are ROSA, LUVA (Laboratory of the University of Virginia), and MCPV-1. They belong to non-mastocytosis donors. ROSA line is available with and without KIT mutations. In vitro culture also employs rodent Mast Cell lines, such as P815 and RBL-2H3.
Application in Cardiovascular Diseases
Typically, mast cell studies focus on their participation in immune response confined to allergy and autoimmune disorders. However, their involvement has been evident in many disorders. For instance, pulmonary hypoxia releases MCP1 in circulation, which triggers mast cells. They initiate inflammation in the vasculature, potentially affecting multiple organs. They also release VEGF and FGF2 for angiogenesis and can modulate blood flow through their inflammatory mediators. These mediators also promote differentiation of fibroblasts and endothelial cells. These findings have propelled the investigation into these cells for cardiovascular disorders.
Atherosclerosis
Occasionally, research groups have documented evidence of mast cell presence in atherosclerotic plaque. Their activation by media containing low-density lipoprotein (LDL) or mast cell-based degradation of high-density lipoprotein (HDL) has alluded to the involvement of these cells in atherosclerosis. These studies encouraged further exploration. Studies showed a link between levels of IgE and reactive oxygen species and hyperlipidemia, which activate mast cells. These cells can trigger apoptosis of endothelial cells, smooth muscle fibers, and macrophages, eventually causing the plaque to rupture. Inhibition of chymase, a key protease, can stabilize plaque, suggesting the significance of these cells in atherosclerosis.
Cardiac Fibrosis
Cardiac tissue contains a mast cell population that increases under stress. Several mast cell-related factors contribute to cardiac dysfunction, such as transforming growth factor (TGFβ), chymase, histamine, renin, matrix metalloproteinases, etc. While TGFβ promotes fibrosis, renin cleaves angiotensinogen into angiotensin I (AngI), chymase converts AngI into AngII. Ang II is a mediator of the renin-angiotensin-aldosterone (RAAS) axis that primarily involves angiotensin converting enzyme (ACE). Intriguingly, studies have demonstrated that a high percentage of AngII in cardiac disorders is ACE-independent. Additionally, histamine antagonists and chymase inhibitors decrease the risk of heart failure. It indicates that these cells are essential for cardiac disease pathology.
Hypertension
Many researchers have also assessed the role of mast cells in hypertension. Triggers for hypertension usually drive vascular injury by activating the immune system. Increased IgE levels, mast cell density, and IL6 were found in hypertensive animal models. IL6 is known to cause endothelial cell dysfunction, aggravating hypertension. Interestingly, IgE neutralization inhibited high blood pressure in mice.
Future Perspectives
After the discovery of mast cells in 1878 by Paul Ehrlich, their research was restricted to specific domains, underestimating their full potential. However, their implication in diverse disorders, especially cardiovascular disorders, is now evident. They have become the targets for developing therapeutics. For instance, chymase inhibitors can reduce cardiac fibrosis and have entered phase I studies. Similarly, many drugs developed for mast cell disorders are now repurposed for cardiac disorders. Omalizumab inhibits IgE interaction with FcεRI by binding to free IgE and has been reported to alleviate cardiac dysfunction, inhibit rising blood pressure, and vascular remodeling. Drugs that suppress mast cell signaling pathways, JAK, SYK, BTK, etc., are also under investigation.
These results emphasize the contribution of these cells to physiological and pathological processes. Kosheeka recognizes the potential of these cells in drug development and, therefore, offers high-quality mast cells in India to promote the formulation of more effective therapies.
FAQ’s
Q- What are mast cells, and where do they originate?
They are vital immune cells that differentiate from myeloid progenitors. They mature in peripheral tissues and play key roles in allergic responses, parasitic infections, and innate immunity.
Q- How are mast cells activated?
Their activation primarily depends on IgE receptors such as the high-affinity FcεRI, which bind IgE and trigger degranulation. Other receptors like toll-like receptors, complement receptors, and the Mas-related-G-protein receptor-2 (MRGPRX2) also respond to diverse stimuli.
Q- Why are mast cells important in cardiovascular diseases?
They contribute to cardiovascular diseases by promoting inflammation, fibrosis, and plaque instability in atherosclerosis. They release factors like chymase, histamine, and TGF-β that influence vascular remodeling, thus increasing the risk of heart failure. Endothelial dysfunction driven by them can aggravate hypertension.
Q- What are the mast cell models for in vitro research?
They can be derived directly from tissues or differentiated in vitro from progenitors. Researchers use specific culture protocols and cell lines like LAD2 and HMC1 to study their functions, activation mechanisms, and roles in various diseases, including cardiovascular disorders.