In the modern fast-growing biomedical research, scientists are increasingly moving towards physiologically relevant models that truly represent the complexity of human biology. Therefore, primary cell cultures, obtained from freshly harvested tissues, have become the standard for authentic cellular behavior.
The Scientific Value of Primary Cell Cultures
Primary cell cultures retain the natural functional and morphological characteristics of their tissue of origin, making them highly useful for research requiring biological accuracy. In contrast to immortalized cell lines—which undergo genetic as well as phenotypic alterations over continuous passaging—Primary Cells shows actual behavior including tissue-specific gene expression, receptor profiles, mechanical properties and metabolic activity.
This is Crucial For:
- Accurate and Anticipatory Toxicological Assessment in drug discovery, where real cellular responses are required.
- Estimating CRISPR-Induced Alterations in gene editing studies, where researchers need primary cells with functional DNA repair mechanisms.
- Regenerative Biology Research, as their true lineage capacity influences the outcome of tissue engineering.
- Research in Immunology, which depends on primary immune cells capable of producing physiologically relevant signaling responses.
In primary cell culture research, these cell systems provide high-fidelity models that bridge the gap between in vitro studies and in vivo validation. Furthermore their short lifespan, contact inhibition and preserved cell-signaling networks make them crucial for clinical studies.
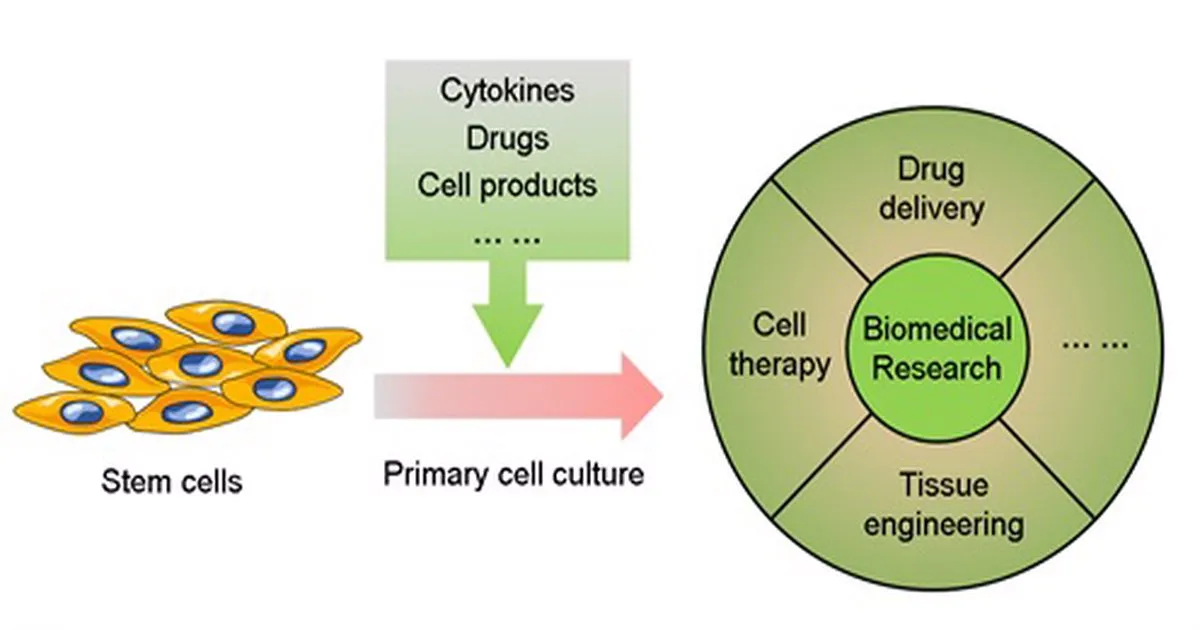
Expert Primary Cell Isolation: Precision in Every Step
Isolating high-quality primary cells demands deep technical expertise optimized tissue handling and standardized workflows. Accordingly, Kosheeka’s Primary Cell Isolation and Culture Services use validated protocols tailored to the specific tissue—blood, bone marrow, adipose, liver, lung, skin, kidney, cartilage, brain, and reproductive tissues.
Our Isolation Methodology Includes:
- Digestion using enzymes like collagenase, dispase, elastase or tissue-specific enzyme blends
- Mechanical separation using gentleMACS or low-shear workflows for the preservation of viability, followed by
- Density gradient centrifugation or lysis of RBCs for blood-derived cells,
- Separation by magnetic and immunoaffinity techniques to obtain high-purity subpopulations, and
- Sterility-controlled workspaces that prevent contamination from the earliest stages.
All batches at Kosheeka go through real time viability tests, phenotypic confirmation tests and sterility tests to achieve consistency and reproducibility.
Culturing Primary Cells: Maintaining Native Physiology
This is the most technically demanding aspects, as these cells require precisely controlled conditions to preserve morphology, functionality, and lineage identity. Consequently, Kosheeka Uses Tissue-Specific Culture Media, growth supplements, ECM substrates, and optimized protocols to maintain the native phenotype for extended periods.
Our Culture Expertise Includes:
- Xeno-free or serum-optimized media, depending on experimental needs
- Defined growth factor cocktails that support proliferation without unwanted differentiation
- ECM coatings (collagen, laminin, Matrigel, fibronectin) that simulate natural microenvironments
- Capability of low-oxygen culturing to replicate hypoxic native tissues
- The use of highly pure water and closed-system workflows for low contamination risks
- Routine mycoplasma, bacterial, and fungal testing for pure cultures
We offer scale-up support, like bioreactor-compatible primary cells for large-volume assays and cryopreservation services using controlled-rate freezing to preserve post-thaw viability and phenotype.
State-of-the-Art Applications Enhanced by Primary Cell Culture Research
Our Primary Cell Isolation and Culture Services support several advanced scientific applications:
Drug Discovery and Predictive Toxicology:
Early-stage screening can be performed using primary hepatocytes, cardiomyocytes, renal epithelial cells, and neuronal cultures, where metabolism and signaling responses are human-like. Thus, this provides far higher predictive power than immortalized lines.
Functional Genomics and Gene Editing:
Working with primary cells ensures gene edits are evaluated in a natural, unmodified background—critical for validating targets and understanding functional outcomes.
Personalized Medicine and Disease Modeling:
Patient-derived primary cells help in target assessment and functional analysis by offering a clean and biologically relevant system.
Engineering 3D Systems and Regenerative Medicine:
Research on organoid development, scaffold engineering and tissue repair is constantly enhanced by primary cells which offers physiologically relevant insights unattainable using transformed cell lines.
Immuno-Oncology and Inflammation Studies:
PBMCs, T-cells, macrophages, and dendritic cells isolated under strict quality control enable precise evaluation of immune pathways and therapeutic responses.
These applications highlight how central primary cell culture research has become to next-generation biomedical innovation.
Customization: Tailored Cell Solutions for Every Project
Kosheeka Offers Fully Customized Primary Cell Solutions to Meet the Exact Needs of Each Project. Customization is Available For:
- Age, gender, health status of donors or their disease status
- Tissue source
- Human or animal models (species-specific)
- Purity requirements for subpopulations,
- Scale-up or batch size,
- 3D culture or co-culture compatibility and
- pre-screening for markers, mutations, or functional attributes.
Each order includes a complete Certificate of Analysis with viability metrics, sterility tests, phenotype verification, morphology images, and donor history—ensuring transparency and reproducibility.
Quality, Ethics and Compliance
Kosheeka adheres to internationally accepted standards of ethical sourcing, documentation, and donor consent. Moreover, each tissue is harvested following approved protocols and can be traced in full accordance with institutional and regulatory guidelines.
Our Quality Systems Include:
- Workflows complying with GMP requirements
- ISO documentation and tracking
- Processes for consistency among batches
- Genetic stability evaluations, and
- Functional and phenotype validation
This robust quality check system ensures that your primary cell cultures are biologically reliable and ethically sound.
Streamlined Solutions That Facilitate Research
Primary Cell Isolation and Culture Services eliminate the need for laboratories to maintain internal tissue procurement teams, isolation expertise, or specialized infrastructure. Accordingly, by partnering with Kosheeka, researchers benefit from:
- Faster experimental timelines
- Higher reproducibility across batches
- Reduced cost and operational risk
- Access to rare and difficult-to-isolate cell types
- Dedicated technical support throughout the project
This efficiency enables research teams to concentrate on discovery, innovation and data generation in place of troubleshooting technical limitations.
Conclusion
Primary cells have become central to the future of biomedical research. Accordingly, whether your focus is drug development, regenerative biology, mechanistic studies, or advanced gene editing, Kosheeka’s Primary Cell Isolation and Culture Services delivers cell solutions that support integrity, consistency, and translational relevance.
Partner with us to unlock the full potential of primary biology and take your primary cell culture research to the next level.
FAQ’s
Q- Can Primary Cells be Customized for Disease-specific Research?
Yes. Primary cells can be sourced from donors with specific conditions enabling more accurate disease modeling and personalized therapeutic research.
Q- What is the Typical Viability of Primary Cells Obtained Through Kosheeka’s Isolation Services?
Kosheeka provides highly viable primary cells after isolation, often greater than 80–90%, depending on tissue type. Each batch is subjected to strict viability testing and phenotypic confirmation to ensure high-quality cultures.