The role of the body’s vasculature has been explored beyond the usual transportation of nutrients, gases, molecules, and cells. Its cellular composition has diverse functions with implications in physiology and pathology. Vascular-derived primary cells modulate blood vessel diameter, participate in angiogenesis, and respond to inflammation.
They also exhibit substantial heterogeneity in their genetic profile and phenotype depending on their tissue association. It indicates the significance of these cells for primary vascular tissue functioning. They are also responsible for the underlying mechanisms of vascular disorders such as atherosclerosis, ischemia, hypoxia, etc. Therefore, several research studies have been focusing on them to discover new therapeutic targets and develop effective medications.
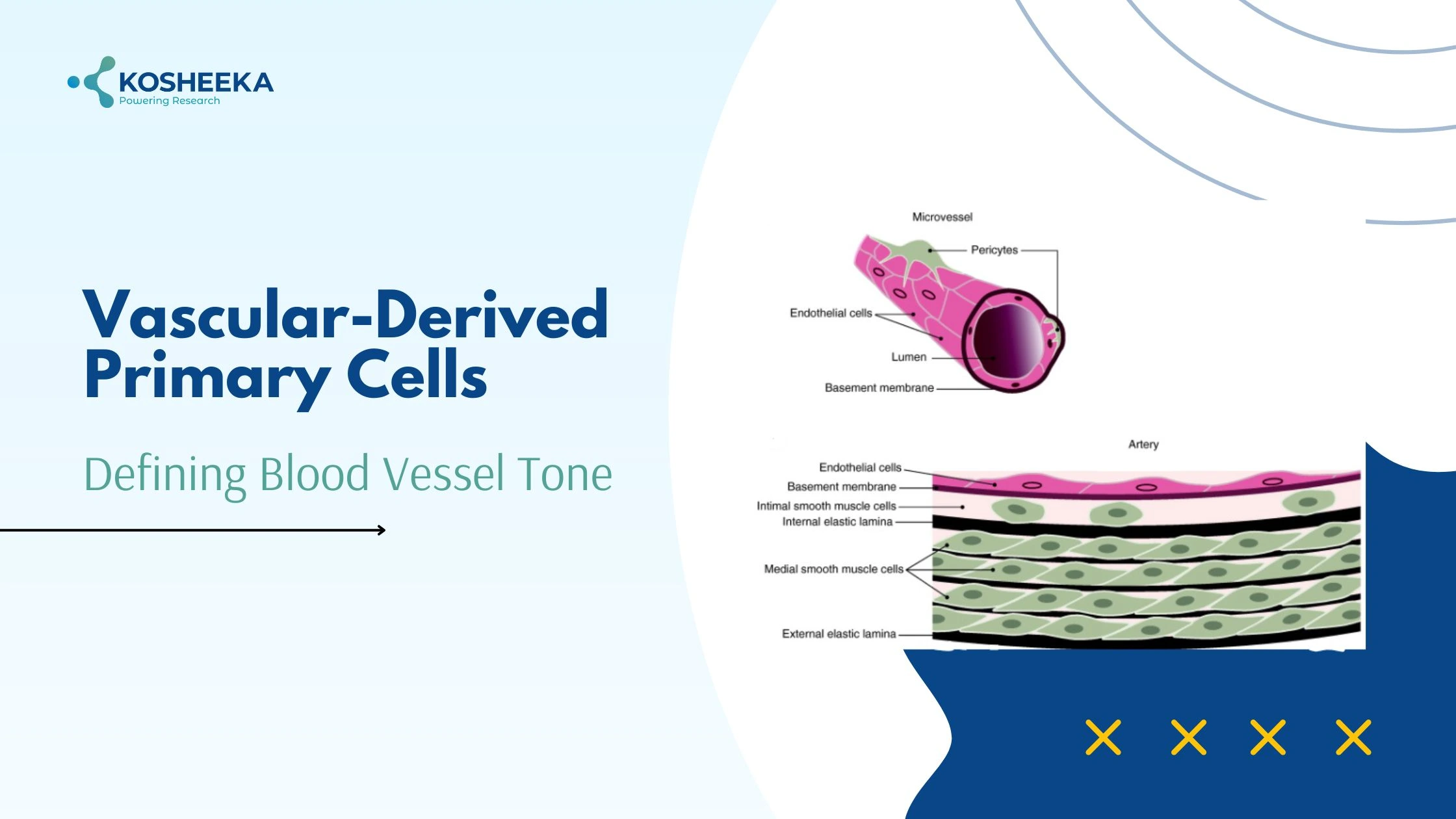
Structure of Blood Vessel
A blood vessel comprises three layers- tunica intima, tunica media, and tunica externa. Intima is the innermost layer on the luminal side, therefore exposed to the blood flow. It contains a single layer of endothelial cells (ECs) and a basement membrane. Tunica media contains vascular smooth muscle in multiple layers and along with elastic fibers. The externa layer consists of connective tissue that has a supply of nerves. Pericytes are another vascular-derived primary cell present on the vessel wall.
Type of Vascular-Derived Primary Cells
Blood vessels contain three different kinds of cell types with their own properties and functions.
Endothelial Cells (ECs): These are the key cells of vessels. Other cell types may not exist at a few locations of vessels, but ECs are ubiquitous in all vessels. They have direct exposure to blood flow and hemodynamics. They incorporate structural changes to alter their permeability depending on the tissue functionality. For example, they form an impermeable layer in the blood-brain barrier but show fenestrations in the kidney and a discontinuous membrane in bone marrow. They also modify their protein expression during various physiological and pathological processes. ECs are vital components of the bone marrow stem cell niche. In fact, mesenchymal stem cells in different tissues are located near the vessels.
Vascular smooth muscle cells (VSMCs): These are the most abundant cell types in vessels. They show fusiform morphology and have invagination to provide large surface area for junctional complexes with the adjacent cells. These cells remain in a quiescent state and exhibit a contractile phenotype by expressing early markers (SM α-actin, SM22-α), intermediate markers (h-caldesmon, calponin), and late markers (SM myosins, smoothelin) of differentiation. Their relaxation and contraction control the vessel diameter and regulate the blood flow. They also produce extracellular matrix by switching their phenotype and grant elasticity to vessels. Studies have also suggested that Vascular Smooth Muscle Cells can de-differentiate and develop characteristics pertaining to macrophages, osteoblasts, and fibroblasts.
Pericytes: Charles Rouget identified pericytes during the 19th century. They have long processes that wrap themselves around the endothelial layer. Their identifying features include perivascular location and the expression of PDGFRβ and CD146. They also express hematopoietic marker CD34 and tissue-specific markers. Their differentiation into lineages- osteoblasts, adipocytes, neurons, and chondrocytes- indicate their multipotency. They are essential for maintaining the stem cell vascular niche in bone marrow and matrix remodeling in the liver.
Vascular Cell Functions
Vascular-derived cells maintain blood flow, blood pressure, and vascular tone. They act as a barrier to the transmigration of molecules and cells. The following are a few functions of these cells.
Blood Flow Regulation: Vessel diameter is vital to blood flow and pressure. All the three cell types govern the changes in vessel diameter. ECs release nitric oxide which binds to its receptor on VSMCs leading to the stimulation of guanylate cyclase and formation of cGMP, which causes muscle relaxation and subsequent vasodilation. Endothelial PGI2 also follows the identical signaling pathway to relax VSMCs. Pericytes has also shown constriction in response to vasoconstrictors. These molecules alter the calcium concentration, constricting pericytes and resulting in vasoconstriction.
Angiogenesis: In case of injury, vessels repair themselves by the formation of new vessels from the previous ones. A process known as angiogenesis. It requires degradation of the basement membrane, migration and proliferation of ECs, tube formation, and vessel stabilization. VSMCs transition from contractile to synthetic phenotype, granting them the ability to proliferate, migrate, and produce collagen. They also secrete matrix metalloproteinases for matrix remodeling. In the initial phase of angiogenesis, pericytes increase their volume and shorten their extensions, while their basement membrane fragments drive their detachment from the vessel wall. The detachment allows ECs to migrate and form vessels. Pericytes invade the new vessels and stabilize them.
Barrier: ECs form a tight barrier in the brain to restrict permeability and transport across the brain. The density of pericytes on this blood-brain barrier is vital to prevent the entry of toxins. ECs also exhibit selective permeability to regulate the transendothelial migration of cells and molecules. VSMCs support the vessel structure and aid in the maintenance of barriers.
Product-Related Queries, Or Partnership Inquiries
Vascular-Derived Primary Cells in Disease
Several vascular disorders, like hypertension, atherosclerosis, and ischemic disorders, originate from the primary vascular tissue.
Ischemic disorders: Ischemia is the decrease in the blood flow to a tissue, leading to apoptosis and tissue failure. It underlies the pathogenesis of diseases such as brain stroke, critical limb ischemia, myocardial ischemia, etc. Shortening of pericytes’ processes is one of the mechanisms of myocardial ischemia. These cells can also transform into collagen-producing fibroblasts and result in fibrosis during chronic kidney injury. Most studies have focused on the role of pericytes in the brain. Early studies suggested that pericyte destruction worsens the disease. However, recent studies have been negating the hypothesis and contributing the vasoconstriction to VSMCs.
Atherosclerosis: Damaged vascular wall releases factors that recruit VSMCs to the tunica intima. They undergo phenotypic switching and proliferate. The loss of differentiation markers progresses into the adoption of features of other cell types. For instance, VSMCs act like macrophages and engulf lipid deposits and necrotic cells. They also show osteoblasts and fibroblast-like characteristics by initiating fibrosis and calcification. All these actions contribute to atherosclerosis.
Thrombosis: In normal physiological conditions, ECs maintain an anti-platelet adhesion surface by releasing tissue factor pathway inhibitors and endocytosis of factor Xa. In case of injury, ECs synthesize PAF and release vWF that favor platelet activation and adhesion to the endothelial surface. Thrombin binds PAR1 and activates signaling pathways in ECs. Together, these pathways transform ECs into a procoagulant surface to form a clot.
Inflammation: ECs release chemoattractants in response to injury to recruit immune cells. They also elevate the levels of cell adhesion molecules required for extravasation of immune cells to the injury site. Furthermore, they loosen their junction complexes to allow immune cells to transmigrate into the underlying tissue. Pericytes also release inflammatory mediators in response to stimuli. These mediators induce neutrophil synthesis and increase their survival.
Conclusion
The various cells that compose primary vascular tissue contribute to its shape and functionality. Every cell participates in different physiological processes and plays diverse roles. Many studies have been prompted by their role in the diseases. Researchers are working to identify their underlying mechanisms. Additional research is ongoing on the development of vascular structure using these cells for transplantation purposes. Kosheeka has an extensive inventory of vascular-derived primary cells from different tissues and species. Our team also offers custom cell isolation services to add precision to your research study.
FAQs:
Q – What are the vascular cells?
These are cells that form vasculature, or blood vessels. They include endothelial cells, smooth muscles, and pericytes.
Q – Which are the key cells in vessels?
Endothelial cells are the basic cellular component of a vessel, since smooth muscles and pericytes are not present in all vessels.
Q – What are the functions of vascular-derived primary cells?
They maintain homeostasis by regulating blood flow, blood pressure, and vascular tone.
Q – What is the function of pericytes?
Pericytes stabilize the blood vessels after their formation. It has been implicated in several ischemic disorders for triggering fibrosis and causing vasoconstriction.