Accounting for close to 8% of the body’s weight is the skin: that is capable of self-healing and renewal. Wounds are the outcome of damaged tissue structure or sin functions. There are several stages in wound healing: hemostasis, inflammation, and proliferative and maturity phases that involve a dynamic interplay of growth factors, cytokines, and chemokines. However, any alteration in the factors or time frames can result in what is termed as chronic refractory wounds and even scarring.
Research is currently exploring new cell sources for skin tissue regeneration given the shortage of skin donor sites-adipose tissue-derived mesenchymal stem cells (ASCs) now enter the stage. There are several advantages of using these cells such as the maintenance of diploid karyotype for 100 generations of culture and their ease of extraction (Li and Guo, 2017). Additionally, the “secretome” or the factors secreted by ASCs have shown to be beneficial for wound healing. These include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and glial cell-derived neurotrophic factor (GDNF) that allow the growth of neurons, basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and angiopoietin that boost new blood vessel formation-what is termed as angiogenesis. This angiogenesis is a very important aspect of wound healing as it allows the recovery of wound blood (Klar and team, 2017).

Early work published in 2009 by researchers Altman and team showed that 6-mm-thick skin wounds on rats were healed by treatment with ASCs on a silk fibroin-chitosan film. The stem cells were found to differentiate into endothelial cells and epithelial cells showing the ability of ASCs to heal wounds.
A Phase I trial (ACellDREAM) published in 2014 reported the suitability and efficiency of ASCs in 7 patients with an advanced stage of the peripheral arterial disease called non-revascularizable critical limb ischemia (CLI) that is a challenge to treat at the clinical setting. The ASCs isolated from abdominal fat showed no abnormalities and were found to induce no adverse effects on patients showing the safety of stem cell therapy. Additionally, an improvement in wound/ulcer healing was observed showing the use of autologous ASC transplantation for this chronic condition.
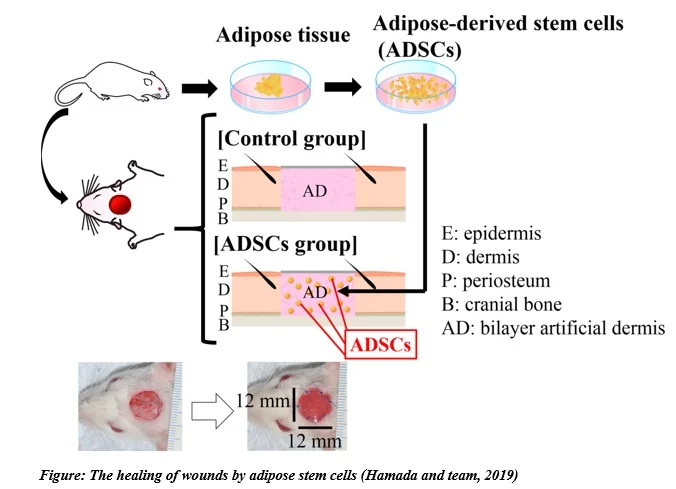
Recent work published in 2019 by Hamada and team showed that ASC administration in rat wound models lowered the average global wound area while increasing the blood vessel density showing the potential of ASCs in wound healing and angiogenesis.
The skin is a vital part of our body and wounds can be a source of both physical and emotional stress. Just like “the eyes are the windows of the soul”, our skin is the window of our health. The safety and efficiency of ASCs in healing the skin shows the way forward for wound treatments. There is no need to step back into the shadow but explore stem cell therapy and come out into the light again.
References:
Li, P., Guo, X. A review: therapeutic potential of adipose-derived stem cells in cutaneous wound healing and regeneration. Stem Cell Res Ther 9, 302 (2018). https://doi.org/10.1186/s13287-018-1044-5
Agnes S. Klar, Jakub Zimoch, and Thomas Biedermann. Skin Tissue Engineering: Application of Adipose-Derived Stem Cells. BioMed Research International. Volume 2017; Article ID 9747010; 12 pages. https://doi.org/10.1155/2017/9747010
Altman AM, Yan Y, Matthias N, et al. IFATS collection: human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27(1):250.
Bura A, Planatbenard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245–57.
Hamada T, Matsubara H, Yoshida Y, Ugaji S, Nomura I, Tsuchiya H (2019) Autologous adipose-derived stem cell transplantation enhances healing of wound with exposed bone in a rat model. PLoS ONE 14(5): e0214106. https://doi.org/10.1371/journal.pone.0214106