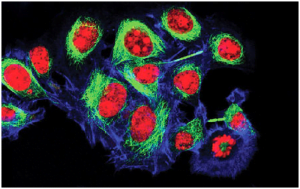
The liver parenchyma comprises two different lineages of epithelial cells- hepatocytes and cholangiocytes. They originate from hepatoblasts during development. Hepatocytes constitute the predominant cells of the liver, playing a vital role in liver functions. Human liver hepatocytes serve as the gold standard for studying hepatic functions and diseases. These cells are involved in inflammation and regeneration, core processes of liver pathophysiology. Therefore, in vitro culture of primary hepatocytes is a fundamental process in hepatic research. However, isolation and maintenance of these cells in the in vitro environment has not been an easy feat. This blog describes the challenges for extraction and culture, along with the strategies to overcome them.
Human Liver Hepatocytes
Human hepatocytes are typically arranged in sheets within the liver. The cytoplasm of hepatocytes is abundant in organelles. Rough endoplasmic reticulum forms proteins for secretion, and smooth endoplasmic reticulum is rich in metabolic enzymes for drugs and cholesterol.
They are polarized with two functionally distinct domains of their plasma membranes. The basolateral domain is in contact with adjacent hepatocytes in the lateral region and vessels at the basal surface. The apical domain forms the bile canaliculus and is responsible for bile secretion. On the other hand, the basolateral domain enables transport.
The gradient of nutrients and oxygen due to proximity to the vascular supply creates different metabolic zones of Hepatocytes. On the other hand, these cells are also distributed into different anatomical regions in lobule- central lobular, periportal, and midzonal- conferring unique characteristics to these cells in each region.
Primary Functions Of Human Hepatocytes
This diversity in cellular properties emphasizes the essential contribution of hepatocytes in liver physiology.
- Bile Synthesis: Bile fluid comprises electrolytes, bile salts, bile acids, water, cholesterol, bilirubin, etc. Hepatocytes synthesize bile and secrete it via canaliculi to enable the digestion and absorption of lipids. The apical domain of these cells is tolerant towards bile and contains different transporters and channels for releasing bile into the canaliculi.
- Drug Metabolism: Hepatocytes contain cytochromes (CYP450, CYP3A4, CYP1, etc.) that metabolize any drug or toxin that enters the body. The reaction occurs in two phases. Phase I converts the drug by oxidation, hydrolysis, and reduction. Phase II conjugates the Phase I metabolite with groups like sulfates and glutathione to increase their water solubility and facilitate their excretion. These functions have led to widespread use of primary hepatocytes in drug screening in pharmacology.
- Nutrient Homeostasis: Hepatocytes clear the blood insulin in case of low blood glucose levels. These cells also internalize iron through clathrin-mediated endocytosis and also promote its release in circulation. They also absorb lipoproteins from plasma and chylomicrons.
- Immune Regulation: The liver receives a huge amount of blood, exposing it to different pathogens. Hepatocytes trigger the innate immune system, leading to inflammation. In case of liver injury, hepatocytes regulate the immune system while also balancing repair and fibrosis.
- Isolation Procedure: Initially, hepatocyte isolation employed mechanical methods that resulted in low cell yield. With time, enzymatic digestion replaced mechanical techniques, considerably increasing the yield. Soon, Seglen optimized the isolation process and introduced the two-step perfusion procedure. It involves circulating calcium-free buffer in the liver, causing irreversible structural damage to desmosomes for disrupting cell-cell contact in the first step. The second step circulates calcium-containing collagenase solution to digest interactions between cells and their extracellular matrix (ECM).
Since tissue samples are more easily available than whole organs, protocols for isolation from samples have been developed. These small tissues do not allow perfusion but typically involve a similar two-step enzymatic digestion procedure. The yield is optimal considering the small size of the tissue. However, the provision of scale-up can enhance the number of cells for downstream applications, offering a much more straightforward and cost-efficient isolation process.
Protocol for Isolation of Primary Human Hepatocytes
The isolation begins with the transportation of the human liver to the laboratory on ice.
- Perfusion Procedure: The equipment for the perfusion process includes a peristaltic pump. Before starting the procedure, perfuse the cannula with a buffer to ensure optimal working of the pump and tubing. The procedure begins with resecting a small tissue and attaching 4-5 cannulas to its blood vessels. Clamp shut the other vessels for better perfusion. The first step of the perfusion procedure is to circulate the tissue with calcium-free EGTA buffer to flush out the blood without clotting. The tissue color change to light yellowish or brownish indicates complete perfusion. The second step of perfusion follows the circulation of collagenase solution. The loss of elasticity or deformation indicates complete digestion.
- Isolation Procedure: It entails placing the tissue on a glass dish and rinsing with ice-cold serum-containing medium to stop the digestion. Shaking the tissue releases cells into the medium. After filtering the cell suspension through serially smaller sieves or mesh, centrifugation at 50-75Xg provides a cell pellet and washing and resuspending the pellet in culture medium results in the isolation of primary hepatocytes.
- Purification: For further purification, place the cell suspension on a density gradient solution. The centrifugation results in a pellet of Human Liver Hepatocytes. Wash the pellet in a buffer and suspend it in a culture medium for culture to obtain a pure population of cells. The polygonal shape and CK18 expression are used to characterize the cells.
The final yield depends on several factors, including digestion time, perfusion rate, donor characteristics, liver sample, etc.
Limitations in Hepatocyte Culture
Primary human hepatocytes are extracted from resected liver tissue or healthy livers that were deemed unfit for transplantation. It limits the quantity of tissue samples available for the isolation process, thus underscoring the significance of hepatocyte culture. However, their long-term culture has been challenging as the cells tend to differentiate into mesenchymal lineages or dedifferentiate. It led to loss of their functions and polarity.
Although cell lines like HepaRG have enabled long-term culture and study of these cells, they do not completely imitate the in primary human hepatocyte functions. Therefore, several studies have attempted to standardize the isolation process for maximal cell yield and viability and optimize the long-term culture conditions.
Hepatocyte Culture
To extend the lifespan of primary hepatocytes in culture, researchers have tried to mimic the in vivo niche of the cells. Therefore, collagen-coated plates are used for in vitro culture to enable cell-ECM interaction. Laminin-rich collagen gel or Matrigel results in more rounded cells showing liver-specific functions for a longer time period. Sandwich culture follows the cultivation of cells in layers by placing another collagen coat on top of the cells. The type of coating underlying and overlaying cells impacts the cellular attributes. But the technique increases the in vitro life span of cells to more than 4 weeks.
Future Perspectives
Hepatocytes are key functional units of the liver, involved in diverse functions. They are a suitable in vitro platform for liver research. However, their isolation and culture remain a daunting task. The isolation procedure has been standardized but requires expertise and sophisticated instrumentation for higher yield. The short retention of tissue functions in culture is also a significant obstacle. The efforts in this direction particularly 3D culture that mimics tissue microenvironment are promoting maintenance of tissue properties for a longer time. Kosheeka offers the human liver hepatocytes at the lowest passage numbers to enhance the fidelity of the research. Our team ensures high purity and viability of cells with consistency between each batch to expedite your research.
FAQ’s
Q-What are hepatocytes?
Hepatocytes are the key functional cells of the liver. They are responsible for vital liver functions, including bile synthesis, drug metabolism, nutrient homeostasis, and immune regulation.
Q-What challenges exist in isolating and culturing primary human hepatocytes?
Isolating primary human hepatocytes from liver tissue is limited by tissue availability and complex procedures like enzymatic digestion. Maintaining them in vitro is difficult because hepatocytes tend to lose their function and polarity by dedifferentiation or transformation into other cell types during culture.
Q-How do hepatocytes contribute to liver inflammation and regeneration?
During liver injury, hepatocytes release pro-inflammatory mediators that activate immune responses but also regulate repair and fibrosis. They not only respond to damage but also influence immune cells to balance inflammation with healing.
Q-What protocols are commonly followed for isolating primary human hepatocytes?
A popular technique is the two-step enzymatic perfusion approach. A calcium-free buffer first breaks down cell-cell connections followed by perfusion with calcium-containing collagenase solution to break down extracellular matrix components to liberate hepatocytes. Viable hepatocytes for culture are then obtained by filtering, centrifuging, and purifying tissues using density gradients. Optimal yields depend on digestion timing, perfusion rate, and donor liver sample quality.