Human skin-derived epidermal melanocytes are responsible for UV protection and skin color. Much investigation has focused on its dermatological applications, pigmentation disorders, and melanoma treatment. Scientists are exploring the signaling pathways behind physiological processes and disease pathogenesis. Current understanding reveals that changes in even the small steps in the pathways can have pathological formation. Therefore, much research is underway to delineate pathways and develop therapeutics.
Human Skin Derived Epidermal Melanocytes
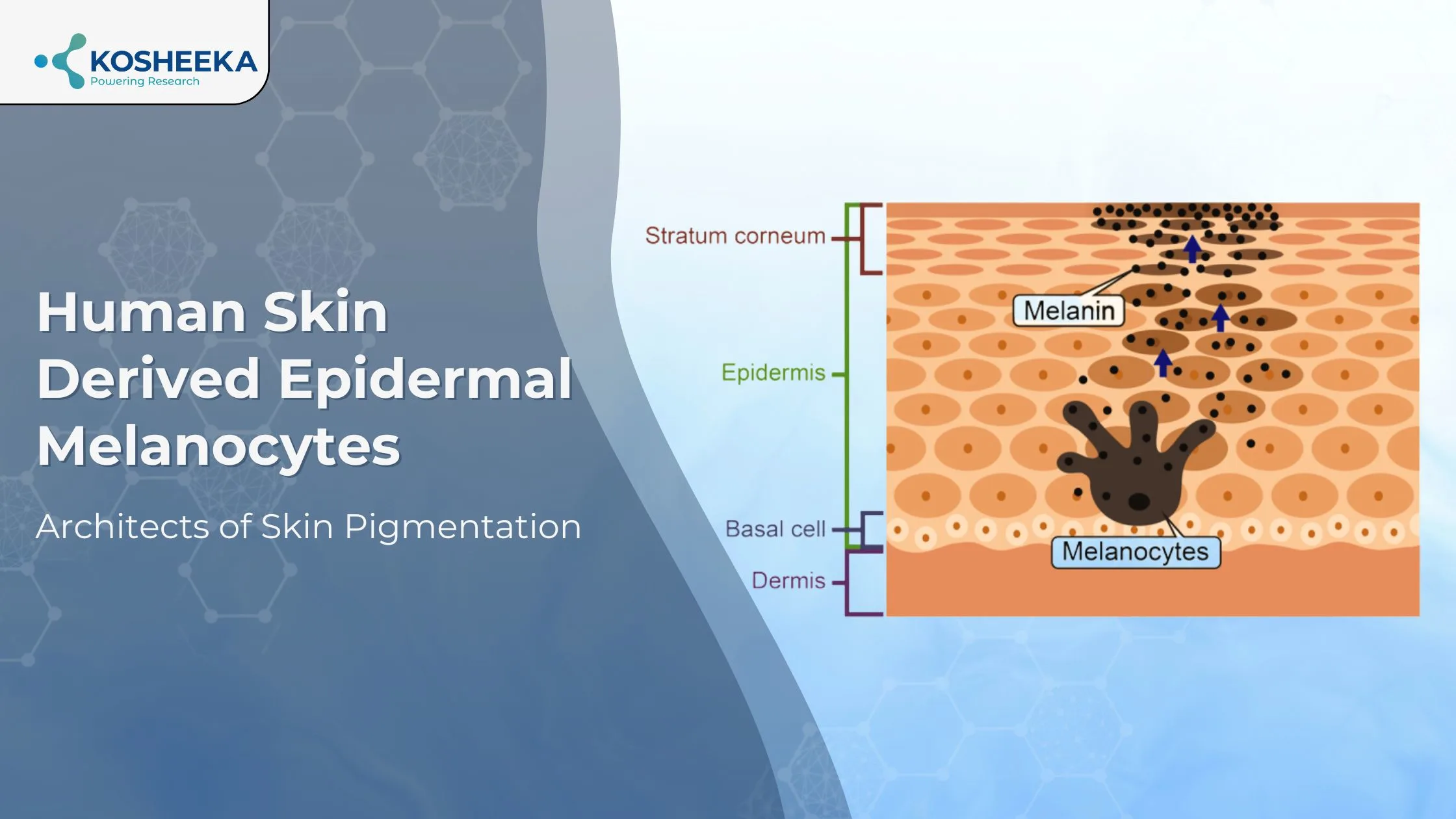
Human skin-derived epidermal melanocytes in adult tissues are melanin-producing cells in the skin epidermis and hair follicles. Melanocytes are also present in the retina, adipocytes, heart, ears, and neurons. However, they differ from epidermal cells in their embryonic origins. Although melanocytes from hair and skin have similarities, they are also distinct in their cell composition and response.
They are oval fusiform-shaped cells with dendritic morphology. They are smaller than keratinocytes and contain melanosomes in their cytoplasm. Melanosomes are melanin-producing organelles. They are identified by their specific protein expression of tyrosinase (TYR), microphthalmia transcription factor (MITF), tyrosine-related proteins (TYRP1, TYRP2), etc.
Origin of Melanocytes
Neural crest cells belonging to the ectoderm germ layer differentiate in later stages into melanocytes. Among neural crest cells, the cranial cells form the dermis of the head, whereas the trunk cells develop the skin epidermis in other regions. The dorsal population of trunk neural crest cells forms skin melanocytes, but recent studies have indicated that ventral cell populations also differentiate into these cells. Schwann cells originating from ventral trunk cells have shown differentiation into melanocytes in vitro in a melanocyte culture medium. The difference in the molecular composition of dermal and epidermal melanocytes supports the claim that these cells arise from two different cell populations. Neural crest cells differentiate into melanocyte precursors- melanoblasts. Melanoblasts then proliferate and migrate to the tissue to transform into melanocytes, requiring Wnt signaling.
Functions of Melanocytes
The primary function of human epidermal melanocytes in adults is the production of melanin that imparts color to the skin and shields it from UV radiation. These cells form two types of melanin pigment- eumelanin, which has a photoprotective effect, and pheomelanin, which generates oxidative stress. Therefore, high amounts of pheomelanin increase the susceptibility to skin cancer.
These cells also participate in immunological function by expressing HLA molecules, presenting antigens to immune cells, and releasing inflammatory mediators such as IL6, TGF-β, IL10, α-MSH, eicosanoids, NO, etc. They participate in complex cross-talk with skin cells—keratinocytes and dermal fibroblasts. Both secrete several factors, such as bFGF, NGF, SCF, NRG1, etc., that regulate melanocyte proliferation, mobility, shape, and pigmentation.
The disruption in melanocyte function can result in hypopigmentation disorders such as vitiligo, oculocutaneous albinism, or hyperpigmentation disorders like lentigo senilis.
Isolation of Melanocytes
The isolation procedure begins with the extraction of a thin section of skin. Its overnight incubation results in the separation of epidermal and dermal layers. Trypsin-EDTA digestion of the epidermis yields two cell populations- keratinocytes and melanocytes. Both can be cultivated in the tissue culture dish. Within a week, melanocytes appear in the culture and can be detached from the culture dish by low-concentration trypsin-EDTA while keeping keratinocytes adhered to the dish.
The melanocytes can be cultured afterward in a keratinocyte growth medium containing 0.2 mM calcium chloride. Further media supplementation can include bFGF, α-MSH, insulin, and triiodothyronine. Some papers suggest low pH for the maintenance of these cells to reduce melanin synthesis and generation of its toxic intermediates for the promotion of cell growth. Furthermore, the addition of factors like stem cell factor, endothelin-1, cholera toxin, etc., can also improve cell survival and maintenance in culture.
Melanocyte Regulation
MITF is the master regulator of melanocyte survival, proliferation, and melanin synthesis. It triggers the expression of melanin-producing enzymes and melanosome-related proteins. Mutations in MITF are linked to melanoma. MITF is, in turn, regulated by proopiomelanocortin (POMC) and opsin (OPN) signaling. In response to UV exposure, keratinocytes and melanocytes secrete high levels of a neuropeptide-POMC. POMC cleavage forms α-MSH that bonds to melanocortin-1 receptor (MC1R) on melanocytes, forming cAMP that triggers MITF expression. OPN are light-sensitive GPCRs on keratinocytes and melanocytes that, upon activation, induce the transcription of TYR, TYRP1, and TYRP2 by cAMP, MAPK, and PKC signaling pathways.
Melanosome Formation
Melanosome formation occurs in four stages (Fig 1). In stage 1, they are similar to endosomes in expressing endosomal markers and showing a spherical morphology. They lack the protein for melanin synthesis. In stage 2, they become elongated with the accumulation of matrix fibrils. This stage also exhibits the presence of melanin synthesis enzymes. In stage 3, their morphology becomes elliptical, and melanin production begins, conferring brown color to melanosomes. At stage 4, melanin covers the fibrils, imparting black color to the organelles and completing the melanosome formation.
Research has suggested that protein trafficking pathways into melanosomes occur via vesicle trafficking from early to maturing melanosomes, from the Golgi apparatus, and through microtubule transport. Mutation in the delivery proteins leads to several disorders, thus demonstrating the significance of the trafficking process.
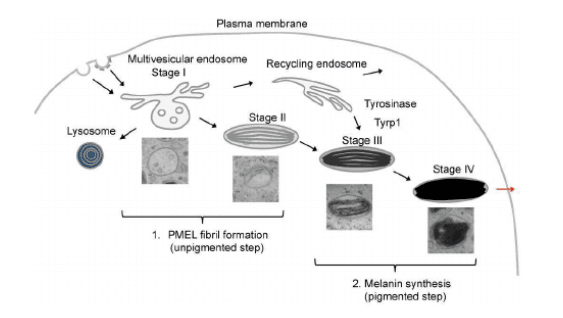
Figure 1. Stages of melanosome formation. (Source- PMID:27589732)
Melanosome Transport
Skin epidermal melanocytes transfer melanin granules into keratinocytes through their dendritic processes. Within the filopodia of dendritic processes, pigment globules encapsulate several melanosomes. After the uptake of melanosomes by keratinocytes, the membrane of globules degrades, and melanosomes accumulate near the nucleus. Although this transfer mechanism is still under investigation, present data alludes to the following different hypotheses for the transport of melanosomes to keratinocytes (Fig 2):
- Keratinocytes phagocytose dendritic processes containing melanosomes.
- Direct transfer of melanosomes by fusion of membranes of keratinocytes and dendritic process.
- Dendritic processes shed melanosome globules. Keratinocyte microvilli capture and absorb them via the protease-activated receptor 2 pathway.
- Melanosomes are released from cells by exocytosis, followed by their uptake into keratinocytes by endocytosis.
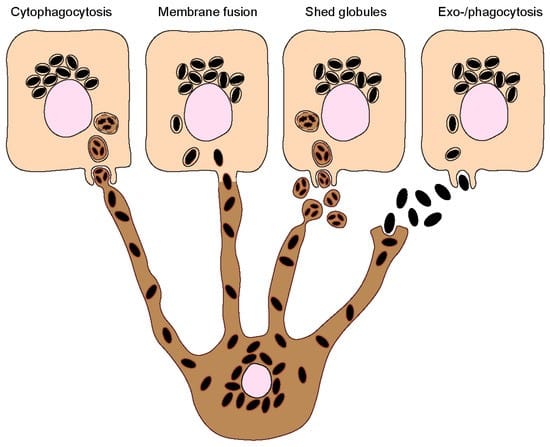
Figure 2. Transport of melanosomes from dendritic processes of melanocytes to keratinocytes. (Source-PMID: 37511054)
Melanogenesis
Melanogenesis or melanin synthesis is a key pathway in melanocytes. The generation of both eumelanin and pheomelanin begins with the conversion of tyrosine into dihydroxyphenylalanine (DOPA) by tyrosine hydroxylase and subsequent formation of dopaquinone from DOPA by TYR. In the absence of cysteine, TYRP1 and TYRP2 convert dopaquinone into eumelanin by a multi-step pathway. Eumelanin exists in brown and black colors, and their ratio defines skin color. Cysteine shifts the reactions towards the formation of pheomelanin, which imparts a red color to hair.
pH of melanosomes plays a key role in melanogenesis. Low pH is crucial for fibril formation during melanosome maturation, whereas pH between 6-7 is essential for the TYR activity. The pH is controlled by membrane transporters such as v-ATPase, OCA2, TPC2, and SLC45A2. Mutation or polymorphisms in these transporters can cause pigment variations.
Product-Related Queries, Or Partnership Inquiries
Melanocytes in Research
Scientists employ melanocytes to develop therapeutics for pigmentation disorders and melanoma. Studies have shown poor response to cancer therapy in melanin-rich melanoma, suggesting inhibition of melanogenesis for better outcomes. Gene editing of TYR has demonstrated improvements in pigmentary disorders. The developmental process of melanocytes is still a subject of investigation. Drug screening on these cells aids in the selection of suitable drug candidates. Research is ongoing to explore pathways for cellular processes such as melanosome biogenesis, melanogenesis, and melanosome transport for drug discovery.
Conclusion
Melanocyte pathway aberrations can drive pathological disorders. There is still much to learn about these cells, and further research can unravel their signaling. Their connection to pigmentation and cancer has stimulated a great deal of investigation. Since there is currently no proven cure for pigmentary illnesses, more research on these cells is necessary. Kosheeka offers human skin epidermal melanocytes from adults to advance research in this field. Our team follows GMP guidelines to ensure cell consistency and reproducibility in results.
FAQs
Q- What are melanocytes?
They are melanin-producing cells found primarily in skin and hair, as well as in the ears, neurons, heart, retina, and other tissues.
Q- What are the functions of melanocytes?
The functions of these cells include melanin synthesis for UV protection, cytokine secretion, HLA molecule expression, antigen presentation, and interaction with other skin cells.
Q- What are melanosomes?
These are organelles that contain melanin synthesis enzymes. They transport to keratinocytes and accumulate near their nucleus for pigmentation.
Q- How does pH affect melanin synthesis?
Melanosome formation requires low pH in the early stages. In the later stages, the tyrosinase enzyme acts at pH 6.8 to synthesize melanin.