Chronic obstructive lung damage (COPD) is a progressive, irreversible damage to the lungs that restricts airflow and impairs breathing ability. Pulmonary epithelial cells play an essential role in immune cell activation and tissue regeneration involved in COPD. Therefore, the development of effective therapies requires an in vitro model for pulmonary epithelium. Lung epithelial cells offer a reliable in vitro model for understanding the physiological process and COPD pathogenesis. This blog outlines the in vitro models for pulmonary tissue and COPD, along with the advanced technologies in pulmonary epithelial cell culture.
Primary Pulmonary Epithelial Cells
Lungs are a complex tissue that is broadly classified into the upper tracheo-bronchial airway and the lower alveolar airway due to their different cellular composition. In vitro studies employ two types of Pulmonary Epithelial Cells in culture- primary bronchial epithelial cells (BECs) to depict the upper airway and alveolar epithelial cells (AECs) to examine the lower airway.
Pulmonary Epithelial Cell Lines
Primary lung epithelial cells from various species have been utilized in research. However, their finite lifespan, limited numbers available after isolation, and donor-based variations have increased the preference for cell lines. Bronchial epithelial cell lines include:
BEAS-2B: It was developed by using viral adenovirus vectors
NCI-H292: It has been derived from cancer tissue,
16HBE14σ : It was created by SV40 T antigen.
A549 is a popular alveolar epithelial cell line derived from non-small lung cancer.
However, they differ from the primary cells in various aspects, restricting the reproducibility of the study results. For instance, the NCI-H292 line shows responses similar to primary cells, but it lacks CYP2A6 and CYP2A13 activity for drug metabolism. Similarly, A549 cell line do not demonstrate tight junctions, characteristic of alveolar epithelium. They also have low levels of drug metabolizing enzymes such as P450, phase 1 enzymes, CYP1A1, CYP2A6, CYP1A2, and CYP2A13. Several research reports have noted that they also vary in sensitivity to environmental stimuli in comparison to primary pulmonary epithelial cells.
Disease Models for COPD
The COPD disease model follows the exposure of cells to components of smoke, cigarette smoke extract (CSE), cigarette smoke particulate matter (PM), or whole smoke. Connection of impingers to the smoke-generating machines, bubbles smoke through the medium in the impingers, forming CSE. Attachment of filter pads to the smoke generator traps smoke particles. Their elution creates PM, which is added to the culture medium for developing disease models. Some studies only add components of smoke, such as formaldehyde and acetaldehyde, to the culture medium. Furthermore, special chambers also expose cells at the air-liquid interface directly to whole smoke.
Air-Liquid Interface (ALI)
After extraction from the tissue, pulmonary epithelial cells de-differentiate in the in vitro environment or submerged culture. Bronchial cells lose their mucociliary phenotype, and the layer they form lacks the pseudostratified morphology and polarized nature. Therefore, lung epithelial Cell Culture requires another approach for the retention of tissue characteristics. Air-liquid interface (ALI) offers an effective alternative.
It entails cultivating cells on transwell inserts/porous membranes roughly comprising 0.4μm pores (Fig 1). After achieving confluence, removal of media from only the apical side of the membrane exposes the layer to air. The resemblance to the in vivo conditions induces and maintains cell differentiation, resulting in the formation of a functional barrier.
Thus, ALI offers a more reliable and accurate model for in vitro studies. It has led to applications of AI in researching several respiratory infections, such as SARS-CoV-2, measuring the impact of air pollutants, and testing aerosolized drugs delivered by inhalation.
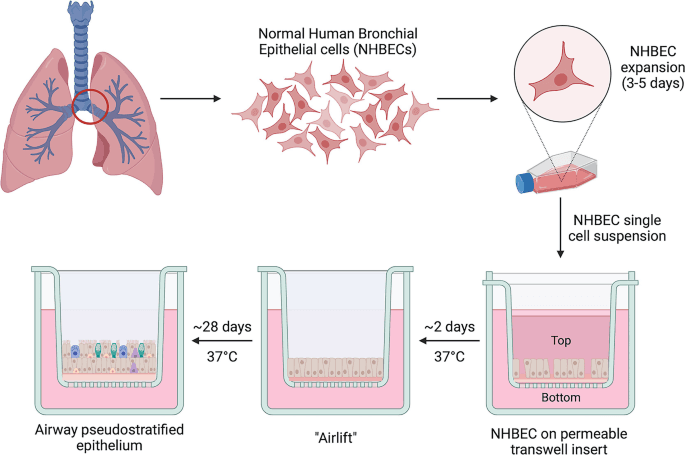
Figure 1. Air-liquid interface technique for culturing human bronchial epithelial cells.
Cell Culture
Several commercially media formulations are available for pulmonary epithelial cells. Supplementation with growth factors such as retinoic acid, transferrin, insulin, hydrocortisone, bovine pituitary extract, EGF, epinephrine, and triiodothyronine results in optimal growth. A mixture of these factors can either be purchased in a ready-made manner or can be developed in-house. Repeated thawing of this mixture can deteriorate the characteristics, so it is advisable to make small one-time use aliquots.
Coating of the cell flask mimics the in vivo basement membrane of lung epithelium. Collagen type I and gelatin are suitable coating materials for BECs and AECs, respectively. Improper coating can lead to insufficient cell attachment. On the other hand, using high concentrations of coating material might change cellular behavior.
Advanced Culture Models
ALI is a suitable in vitro culture model for pulmonary epithelium. However, the recent advances, especially in the field of 3D tissue culture, have also extended to pulmonary cell culture. It enhances the accuracy of the study with better reproducibility in humans.
Organoids
Organoids represent spherical structures that develop from self-assembled cells in a culture. These cells generally include Stem Cells that differentiate into tissue cells under specific culture conditions. Furthermore, epithelial cells from COPD patients can also construct lung organoids. These organoids can be categorized as bronchiolar organoids, alveolospheres, bronchoalveolar organoids, or tracheospheres depending on their cellular composition (Fig 2). They mimic the cell-cell interactions that increase the understanding of tissue physiology.
Due to the self-renewal capacity of organoids, they can be maintained in culture for longer periods. They also aid in the study of differentiation and development of the organ. The drawback of organoids is their lack of circulatory and immune systems. However, their co-culture with these cells can address this concern. Therefore, organoids have found applications in understanding the pathogenesis of COPD and other lung diseases.
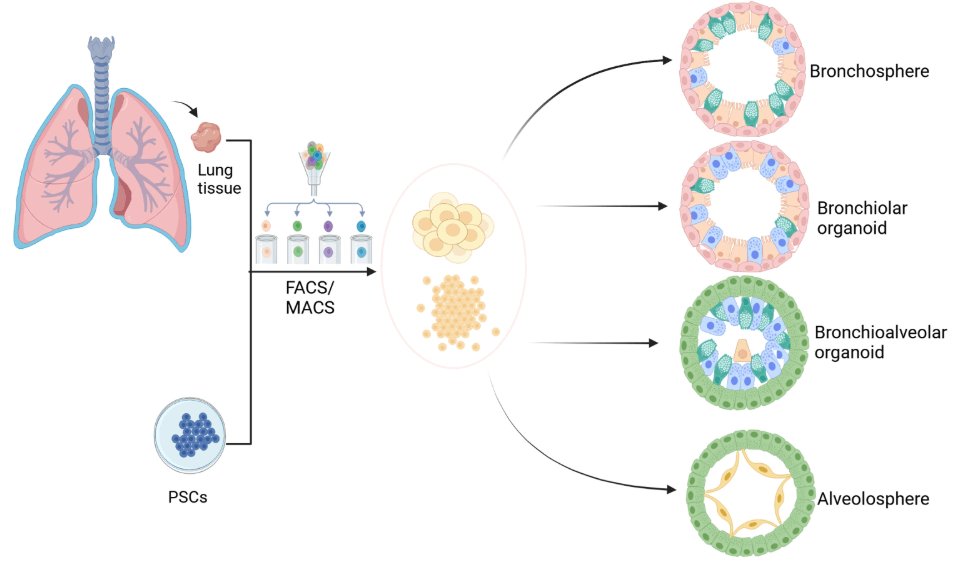
Figure 2: Different types of lung organoids derived either from pulmonary cells or induced pluripotent stem cells (PSCs).
Lung-on-chip
Organ-on-a-chip models have drawn considerable interest for better representation of the in vivo tissue microenvironment. Lung-on-a-chip integrates various pulmonary cells and extracellular matrix to account for tissue complexity and cellular dynamics. It employs a polydimethylsiloxane (PDMS) due to its flexibility, transparency, and biocompatibility to create the chip. A porous PDMS membrane separates the chip into the upper and lower chambers. Coating on both sides of the membrane allows attachment of epithelial and endothelial cells in the upper and lower chambers, respectively.
The chip simulates the breathing motion and vascular flow. Air flows in the upper chamber, and medium flows in the lower chamber. The shear stress deforms the chambers, mimicking the cyclic tissue stretching during the breathing process (Fig 3). This model enables the study of epithelial barrier functions, permeability, and gaseous exchange.
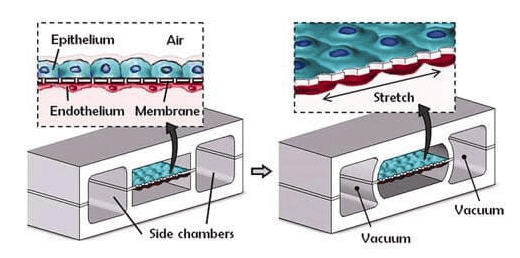
Figure 3: Lung-on-chip device showing stretching of PDMS membrane. It contains cells in the middle chamber and two side chambers.
Product-Related Queries, Or Partnership Inquiries
Conclusion
The 3Rs of scientific research minimize the use of animal models by reduction, replacement, and refinement. It always drives the search for more physiologically relevant models. While submerged culture is the routine in vitro practice, it is not suitable for pulmonary epithelial cells, for they de-differentiate in culture. ALI culture techniques imitate the in vivo characteristics that induce cell differentiation and provide better representation. Lung organoids and lung-on-chip have created their distinct niche. Organoids can utilize cells from COPD patients, paving the way for personalized medicine. On the other hand, lung-on-chip simulates airflow, lung stretching during breathing, and vascular flow. For advancing COPD research, Kosheeka delivers pulmonary epithelial cells after thorough characterization. Its team of experts also offers cell culture guidance for enhancing cell survival and proliferation.
FAQ’s
Q- Which cells are suitable for in vitro research on COPD?
Bronchial and alveolar epithelial cells are used for COPD research. They are exposed to cigarette smoke in different ways to create disease models for COPD.
Q- Are cell lines available for COPD research?
Yes, cell lines like BEAS-2B and A549 are frequently used in tissue culture, but they differ from primary cells in some aspects that can affect the reproducibility of results.
Q- Bronchial epithelial cells are dedifferentiated in culture. What to do?
These cells tend to dedifferentiate in an in vitro environment. Employing the ALI technique for culture will maintain the cell differentiation.
Q- What are the applications of lung-on-chip models?
These models integrate airflow and vascular flow, along with mimicking the shear stress from air. Therefore, they are used to study epithelial barrier properties and gaseous exchange.