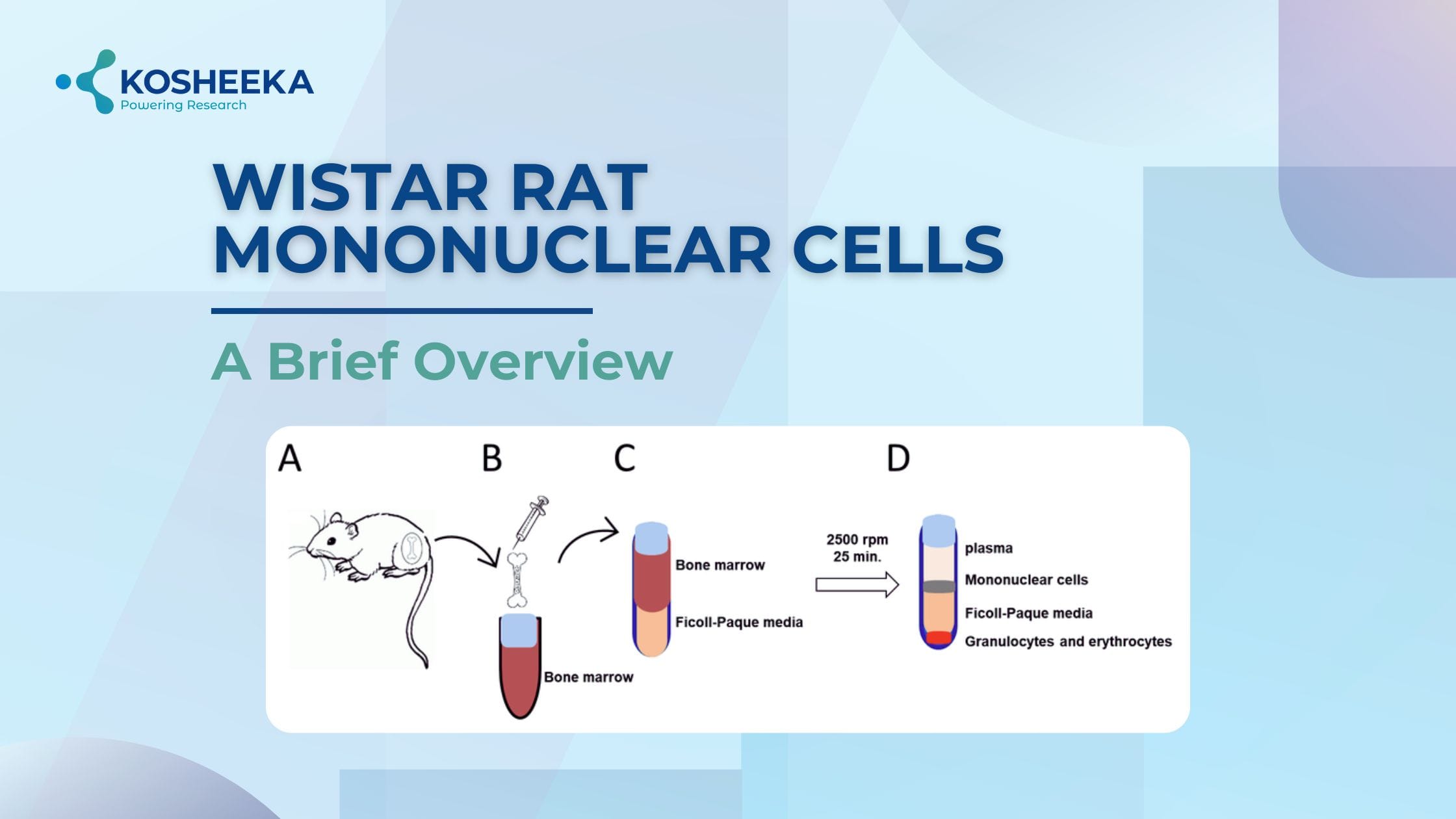
Rat bone marrow mononuclear cells contain different immune cells. They are implicated in disease pathophysiology by their immune cell response and the alterations in their genetic composition. Their use as biomarkers for diagnosis and prognosis has been spurred by changes in their genome and proteome. They also contain progenitors that have the ability to develop into a variety of tissue cells, which supports their application in regenerative medicine. Their use in biomedical research has increased significantly due to their many applications, facilitating the development of new drugs.
Mononuclear Cells (MCs)
Mononuclear cells are immune cells or leukocytes with a single round nucleus. Depending on their source, they are referred to as bone marrow mononuclear cells or peripheral blood mononuclear cells (MCs). They include various leukocytes- lymphocytes (T lymphocytes, B lymphocytes, Natural Killer cells), monocytes, and dendritic cells (DCs). In contrast, other immune cells are known as polymorphonuclear leukocytes, referring to their multinucleated morphology.
Origin of MCs
All leukocytes, including mononuclear cells, originate from hematopoietic stem cells in the bone marrow. They differentiate into myeloid and lymphoid progenitors. Lymphoid progenitors form lymphocytes, whereas myeloid progenitors differentiate into monocytes and dendritic cells. Myeloid progenitors also form neutrophils, basophils, and eosinophils, which are the polymorphonuclear leukocytes.
Isolation of MCs
Peripheral blood, bone marrow and umbilical cord blood are the sources of MCs. The isolation procedure employs different methods.
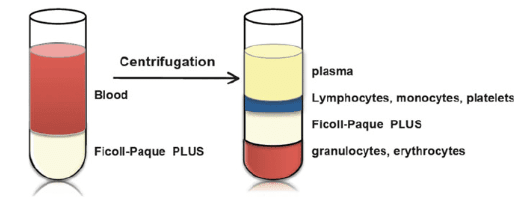
Density Gradient Centrifugation: It exploits the difference in densities of various blood cells. Briefly, the bone marrow fluid or whole blood is collected from the individual and diluted in a buffer (Fig 1). It is then layered on top of a density gradient medium with a 1.077g/mL density, such as Ficoll-paque or Histopaque. After centrifugation, platelets, erythrocytes, and polymorphonuclear leukocytes settle at the bottom, whereas the MCs concentrate at the interphase. This process is cost-effective, requiring no sophisticated instruments. But at the same time, it is also time-consuming and laborious. With no possibility of automation, variability could occur in different batches. Another drawback is the low cell recovery and contamination by other cells.
Cell Preparation Tubes: Tubes like SepMate save time by adding the density gradient medium in the collection tube and allowing rapid centrifugation after gathering the sample.
Cell Sorting/Separation: Another technique is antibody-based separation. It uses antibodies for MCs conjugated to magnetic beads and separates them. This technique yields a highly pure cell population. In a similar manner, flow cytometry activated sorting (FACS) utilizes fluorescent dye-conjugated antibodies and sorts MCs in a flow cytometer.
Leukapheresis: It flows the blood through a machine that separates the MCs by centrifugation and passes the rest of the blood to the patients. The cell number obtained is higher in this technique.
Characterization of MCs
Flow cytometry can characterize the isolated MCs based on their size and granularity using forward scatter and side scatter, respectively. It can provide details of contamination by erythrocytes, platelets, and polymorphonuclear leukocytes. Additionally, the technique can also analyze cell surface markers for different cellular components in MCs:
T Lymphocytes
They drive the cell-mediated immunity of the adaptive immune system. They require antigen presentation on the cell surface of infected or immune cells for activation. T lymphocytes mature in the thymus and are classified into helper T lymphocytes (CD4+), cytotoxic T lymphocytes (CD8+), and regulatory T lymphocytes. Helper T lymphocytes activate other leukocytes, whereas cytotoxic T lymphocytes directly destroy the cells that activated them. Regulatory T lymphocytes prevent the immune response against the self. Therefore, the presence of T lymphocyte markers such as CD3, CD4, and CD8 can aid in MC characterization.
B Lymphocytes
B lymphocytes are the antibody-secreting leukocytes and constitute the humoral immunity of the adaptive immune system. After activation, they undergo somatic hypermutation and affinity maturation. It changes the structure of the antibody, conferring it different specificities and affinities for the pathogen. B lymphocyte markers- CD19 and CD20 are suitable for identifying the MC population from other cell populations.
Monocytes
Monocytes are present in the peripheral blood and also reside in tissues. They differentiate into macrophages and DCs. The key function of macrophages is to engulf the dead cells or debris by phagocytosis, as well as destroy pathogens. The M1 and M2 macrophages participate in immune and repair responses, respectively. Depending on the tissue condition, the interconversion occurs between the subtypes. DCs have cytoplasmic extensions similar to neuronal dendrites. After interaction with an antigen, they undergo a maturation process to present antigenic peptides to T lymphocytes. Their marker CD14 can detect MCs.
Natural Killer (NK) Cells
They interact with infected or abnormal cells and destroy them. The NK cell receptor recognizes surface markers on normal cells and relays inhibitory signals that suppress NK cell’s cytotoxic activity against normal cells. Lack of cell surface marker due to pathogen infection or tumor does not prompt these inhibitory signals, resulting in NK cell cytotoxicity against them. Natural Killer cell response is particularly beneficial when the target downregulates the surface markers that aid in recognition by T lymphocytes. The NK cell marker, CD56, can be employed to assess the MC population.
Wistar Rat Mononuclear Cells
Wistar rat peripheral blood MCs or bone marrow MCs are used in research for several reasons:
Immunological Studies: Rat bone marrow MCs contain leukocytes, and the Wistar rat, a well-characterized outbred strain, provides a reliable model for studying immune responses, inflammation, and autoimmune diseases.
Disease Modeling: Wistar rats are suitable models for diseases like diabetes, cancer, and cardiovascular disorders. Peripheral blood MCs from these rats can be used to investigate the underlying mechanisms of diseases and the immune response against them in order to explore potential treatments.
Toxicology and Drug Testing: Wistar rats are a standard choice in toxicology due to their genetic diversity and physiological similarity to humans. MCs can be used to assess the effects of drugs or toxins on the immune system, aiding in safety and efficacy evaluations.
Genetic Stability: As an outbred strain, Wistar rats maintain genetic variability, which can mimic human population diversity better than inbred strains, making their MCs suitable for studies requiring broader genetic representation.
Availability: Animal models such as Wistar rats are readily available. Standard isolation techniques of Wistar rat MCs are already present. Additionally, a number of studies on these MCs have described their biochemical mechanism, which aids in future study and guarantees experiment reproducibility.
Scientists choose Wistar rat MCs based on the specific research needs, taking into account the relevance of the model, experimental consistency, and translational potential.
The Concluding Statement
Wistar rats have been an optimal animal model for toxicological, pharmacological, and investigative studies. Their easy availability and well-studied properties have promoted the use of MCs from Wistar rats. They provide insights into autoimmune diseases, drug safety, and efficacy. The developments with Wistar rat MCs are more relevant to humans than the frequently used mouse models. Kosheeka provides the Wistar rat bone marrow MCs and peripheral blood MCs to elevate your research to the next level. Our team isolates the product by standardized protocols and characterizes it thoroughly to enhance the reproducibility of your experiments.
FAQs
Q: What are mononuclear cells (MCs)?
These are immune cells with a single nucleus found in peripheral blood. They are also present in bone marrow and umbilical cord.
Q: Why are Wistar rat MCs utilized in research?
The properties of Wistar rat MCs are well researched which provide insights into the experimental data. Additionally Wistar rats are easily available and incorporate the genetic diversity similar to humans, facilitating the use in research.
Q: What is the use of MCs?
The immune response of MCs and the change in their genetic profile can enhance the understanding of the disease pathology. Moreover, the changes in immune response can be exploited as biomarkers for diagnosis and prognosis.
Q: What is the difference between mononuclear and polymorphonuclear leukocytes?
Mononuclear cells contain a single nucleus whereas polymorphonuclear leukocytes contain more than one nucleus. The former contain lymphocytes, monocytes, and dendritic cells, whereas the latter comprises neutrophils, basophils, and eosinophils.