Stem cell research is a fast-growing field due to its applications in regenerative and restorative therapies. Mesenchymal stem cells (MSCs) are an essential component of stem cell therapy owing to their effectiveness in several incurable disorders. Their acquisition has prompted tremendous research. It is often associated with invasive procedures when extracted from bone marrow or adipose tissue. The collection of umbilical cord MSCs is limited to childbirth. Dental pulp-derived stem cells, however, have introduced a new source of MSCs. Tooth is a medical waste and is not dependent on certain medical situations. Therefore, using dental pulp as a source for MSCs is a more appealing alternative.
Dental Pulp Derived Stem Cells
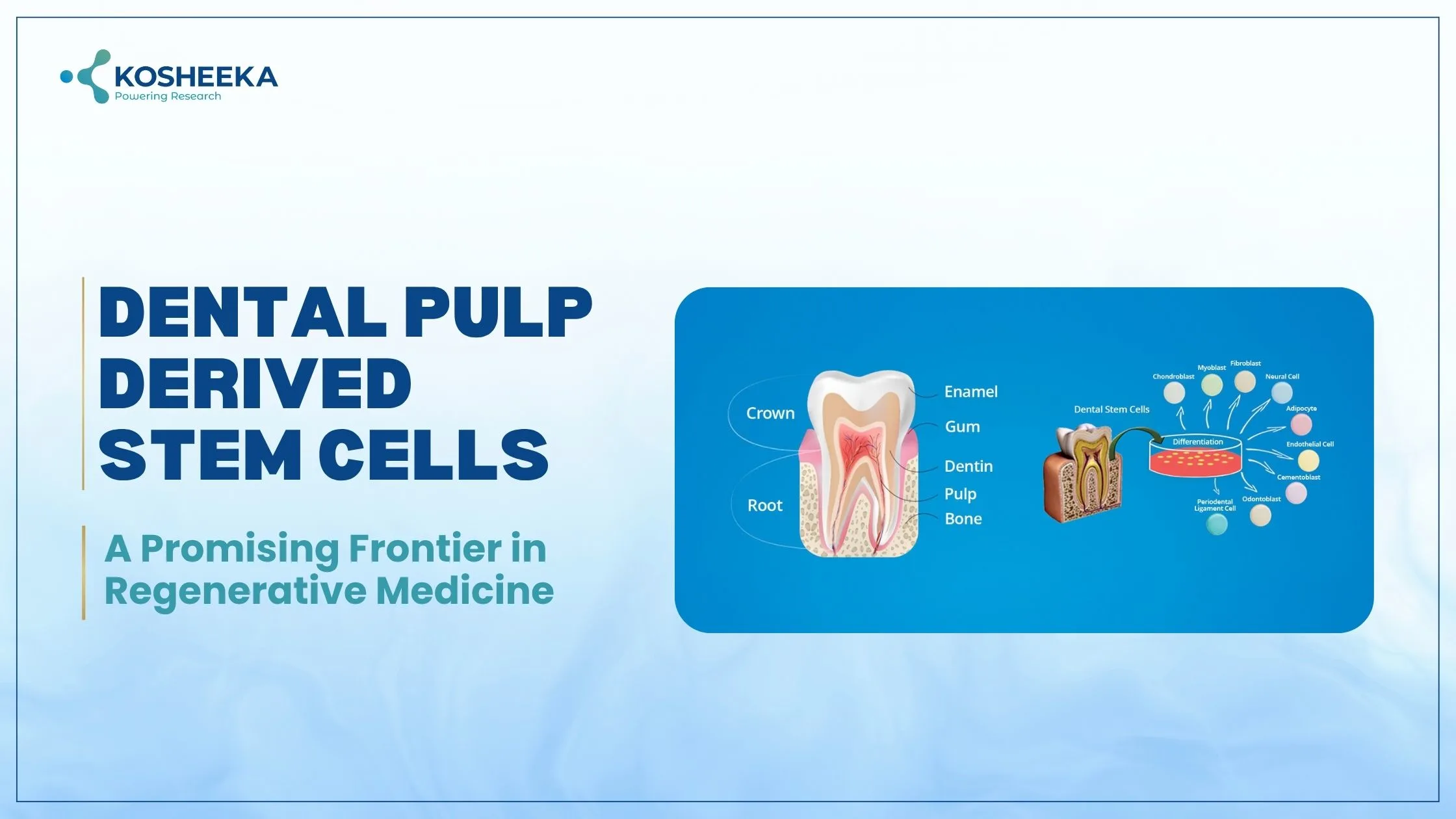
Dental Pulp is a connective tissue present within the mineralized chamber of teeth. It contains MSCs, nerve cells, blood vessels, and lymphatics. The vessels and nerve fibers enter the pulp by opening at the tip of the root. Dental pulp-derived mesenchymal stem cells (DPMSCs) share many similarities with bone marrow MSCs (BMMSCs) and several differences as well. For example, the expression of 3G5 is higher in DPSCs than in BMMSCs.
The presence of markers such as GFAP, P75, HNK-1, S100, and Nestin indicate their originating from cranial neural crest cells. Therefore, these cells have the ability to differentiate into neurons, β-islet cells, endothelial cells, and cardiomyocytes. Furthermore, they can form adipocytes, osteoblasts, and chondrocytes similar to BMMSCs.
Dental Pulp Stem Cells Isolation
The Source: The initial isolation procedure employed third molars as the cell source. However, exfoliated deciduous teeth, also known as baby teeth, also proved to be excellent sources. Scientists have emphasized the details of the source for DPSCs. Stem cells from baby teeth are referred to as SHEDs, and the ones with deciduous origin are SCDs. SHEDs reportedly have a higher proliferation rate with more pluripotency and neuroectodermal markers.
The Isolation Procedure: It follows two different methods. The earliest isolation process utilized enzymatic methods. It requires dissection of the tooth to retrieve pulp tissue and the subsequent digestion of the pulp in collagenase and dispase. On the other hand, the explant method uses the pulp tissue and culture it in the growth medium. Cells gradually transfer to the medium.
Characterization: The procedure follows the analysis of cell surface markers. Due to their similarities to BMMSCs, their characterization follows the same process as that of BMMSCs. For example, evaluation of by the positive expression of BMMSCs markers — CD29, CD90, CD105, CD73, STRO-1, CD44, CD146, CD166, and CD271 and the negative expression of hematopoietic markers. Additionally, the functional characterization assesses the differentiation of DPSCs into adipogenic, osteogenic, and chondrogenic lineages by different assays.
Immunomodulatory Properties
Several reports have emerged highlighting the immune modulation characteristics of DPSCs. In vitro co-culture of DPSCs with T lymphocytes has demonstrated arrest of lymphocyte proliferation at the G0/G1 phase of the cell cycle. The conditioned medium of DPSCs also inhibited the proliferation of peripheral blood mononuclear cells. DPSCs also release IL-10 and TGF-β that lead to the proliferation of regulatory T lymphocytes. They also suppressed the secretion of inflammatory mediators in macrophages in a co-culture model.
Fate of Stem Cells
Epigenetic factors determine the stem cells’ fate, including histone deacetylation and DNA methylation. Histone deacetylases (HDACs) are enzymes that regulate chromatin structure and, therefore, its subsequent gene expression. HDAC inhibitors valproic acid in DPSCs improved the expression of bone-related gene profiles and matrix formation. They also formed bone tissue after subcutaneous transplant in mice. Similarly, trichostatin A, another HDAC inhibitor, improved their renewal and migration.
Clinical Applications
A small amount of DPSCs are available after isolation, which can impede its future applications. According to studies, they may be cultured for an extended period of time without losing their morphology or stem cell markers. They also have low levels of HLA-II DR expression, which suggests a reduced likelihood of immunological responses following infusion. Their applications have been investigated as a result of both benefits.
Neural Regeneration
DPSCs belong to neural crest ontogeny and thus have demonstrated the ability to differentiate into neurons. They also generate neurotrophic factors to promote neuron survival. Animal studies have shown that the graft of DPSCs in the spinal cord resulted in increased quality of motor neurons, thereby proving their potential for various neurological disorders.
Retinal Regeneration
Research has indicated the role of DPSCs in retinal disorder by their capability to express neurotrophins such as neurotrophin-3, brain-derived neurotrophic factor, and nerve growth factor after co-culturing them with rat retinal cells. They have expressed markers of photoreceptor cells, supporting their capacity for visual restoration.
Bone Regeneration
The osteogenic capacity of DPSCs has promoted their use in bone tissue engineering. It employs three-dimensional (3D) scaffolds that are osteoinductive in nature to produce mineralized bone tissue. Implantation of DPSCs with a porous scaffold of hydroxyapatite and tricalcium phosphate showed the formation of well-mineralized bone tissue with partially developed hematopoietic tissue. The addition of certain growth factors like bone morphogenetic protein-2 (BMP2) exhibited osteo-inducibility. Additionally, the differentiation of DPSCs into osteoblast and their subsequent culture on a scaffold of collagen, hydroxyapatite and poly-lactic-co-caprolactone stimulated osteoblast proliferation. Therefore, DPSCs can effectively treat orthopedic disorders.
Vascular Regeneration
DPSCs secrete vascular endothelial growth factor (VEGF) and can differentiate into endothelial cells that have the potential to repair vascular damage. In rat models of infarcted myocardium, they improve the infarct size and increase the blood vessel quantity. Their neovascularization ability was evident by the formation of tube-like structures on Matrigel using DPSCs. Their infusion in limb ischemia models augmented blood flow and vessel formation. Their ability of angiogenesis can be particularly beneficial in disorders such as stroke and cardiovascular disorders.
Liver Transplant
In vitro studies have shown that DPSCs can differentiate into hepatocytes, integrating their morphology and functions. The differentiated cells were able to perform hepatic functions such as glycogen storage, urea production, etc. Thus, DPSCs can be employed in the future to treat liver diseases.
Diabetes Treatment
Several reports have described the differentiation of DPSCs into β-islet cells. They showed glucose-dependent insulin secretion along with expression of β-cell development genes like duodenal homeobox-1, insulin, etc. They formed islet-like clusters that were able to reverse hyperglycemia in diabetic mouse models.
In a Nutshell
DPSCs have provided a novel and easily accessible source for MSCs. The teeth being a medical waste can even pave the way for DPSCs banking in the future. They are different from other MSCs due to their ontogeny, which imparts the characteristics to differentiate into neuronal cells. Presently, the investigations are more focused towards delineating their differentiation mechanisms and their potential therapeutic applications. With the growth of MSCs in clinical trials, DPSCs might soon witness their clinical use. Kosheeka delivers these DPSCs after extensive testing and characterization to empower your stem cell research.
FAQs
Q: Where do dental pulp stem cells (DPSCs) reside?
They are present in the pulp tissue- a soft connective tissue inside the teeth.
Q: Where do dental pulp stem cells originate from?
They originate from neural crest cells and therefore have the ability to differentiate into neurons, pancreatic, and liver cells.
Q: What are the markers for dental pulp stem cells?
Since they are similar to mesenchymal stem cells (MSCs), the markers and assays to identify DPSCs are also identical to that of MSCs. Therefore, markers of DPSCs include CD90, CD44, CD271, STRO-1. Additionally, neural markers like GFAP, S100, P75, etc., can also be estimated.
Q: Do DPSCs also have immunomodulatory properties?
Yes, like MSCs, DPSCs also have immunomodulatory properties that can suppress the proliferation of immune cells and stimulate the regulatory T lymphocytes.