Mesenchymal stem cells for TB
Not only is tuberculosis (TB) among the top 10 causes of death in the world but it also is the leading cause of death due to a single infectious agent! Though curable and preventable, close to 10 million people (5.7 million men, 3.2 million women and 1.1 million children) contracted the disease in 2018 regardless of age groups and countries. These figures accounted for a total loss of 1.5 million (inclusive of 251000 HIV patients) lives across the world. India leads in the high TB burden countries with the others being China, Indonesia, the Philippines, Pakistan, Nigeria, Bangladesh and South Africa (WHO, 2018).
Given the alarming figures, treatment options are being actively sought after in order to meet the Sustainable Development Goal of ending the TB epidemic by 2030.
One of the major challenges of the pathogen Mycobacterium tuberculosis (Mtb) is its multiple evasion schemes to escape detection by the immune cells to multiply and spread infection. Mtb is internalized by macrophages within an immature membrane-bound phagosome to be processed and presented to CD 4 cells. These cells secrete Th1 cytokines such as IFN-γ and TNF-α to activate the macrophage to secrete nitric oxide and superoxide to cause the killing of the pathogen. One of the major ways the pathogen evades the immune response is the inhibition of phagosome-lysosome fusion within macrophages to prevent the killing mechanisms. This causes the organism to persist in a dormant state in macrophages making it a challenge to kill them.
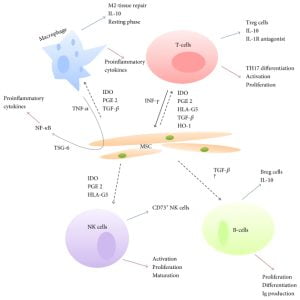
Mesenchymal stem cells (MSCs) have been shown to express Toll-like receptors (TLRs), NOD2 and RIG-I. Additionally, research has repeatedly shown the ability of these cells to modulate the immune system especially the M1 and M2 phenotypes of macrophages and dendritic cells.
An open-label phase 1 safety trial (German Clinical Trials Registry, number DRKS00000763) published by Skrahin and team (2014) showed the safety of single-dose autologous bone marrow-derived MSCs in patients with MDR/XDR tuberculosis.
The WHO estimates that 78% had MDR-TB (multidrug-resistant TB) with 484 000 new cases with resistance to rifampicin – the most effective first-line drug! Analysis by scientists Skrahin and team (2016) of the data from 72 M/XDR-TB patients who received chemotherapy in Belarus was done. Of these, 50% of patients received autologous mesenchymal stromal cells while the remaining were controls. The culture conversion rates at 2 and 6 months, radiology at baseline and 8 months and final treatment outcomes revealed that 81% of the cases showed successful outcomes with radiological improvement and a better prognosis than that of the controls showing the promise of MSCs in improving the outcome for MDR-TB: a major public health crisis.
Khan and team (2017) showed that Mtb could be internalized by two types of scavenger receptors: MARCO (macrophage receptor with a collagenous structure) and SR-B1 (CD36) on the MSCs. Additionally, the live Mtb did not multiply in MSCs while treatment with rapamycin induced autophagy to boost the intracellular killing of the pathogen. This phagocyte-like function of MSCs from the human bone marrow (BM-MSCs) and umbilical cord (UC-MSCs) coupled with their advantages of easy availability shows the way ahead for stem cell-based therapy for TB.
References:
https://www.who.int/news-room/fact-sheets/detail/tuberculosis
Skrahin, A. et al. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med 2, 108–22 (2014).
Aliaksandr E. Skrahin, Helen E. Jenkins, Hennadz Hurevich, et al. Potential role of autologous mesenchymal stromal cells in the treatment of multidrug and extensively drug-resistant tuberculosis. European Respiratory Journal Sep 2016, 48 (suppl 60) PA1919; DOI: 10.1183/13993003.congress-2016.PA1919
Khan, A., Mann, L., Papanna, R. et al. Mesenchymal stem cells internalize Mycobacterium tuberculosis through scavenger receptors and restrict bacterial growth through autophagy. Sci Rep 7, 15010 (2017). https://doi.org/10.1038/s41598-017-15290-z.