Introduction
Regenerative medicine restores, repairs and replaces the damaged cells/tissues. Stem cell research plays a primary role in regenerative medicine, involving the development of stem cell treatments. In biomedical research, mice are the primary mammals for research purposes. Mouse models used for translational study due to their similarity to human disease, shorter life span, and quick breeding capability. Mouse Neural Stem Cells (MNSCs) serve as a critical bridge between neuroscience discoveries and application of human clinical trials.
Biological Characteristics of MNSCs
Mouse neural stem cells are multipotent cells that can differentiate into various cell types that form the central nervous system (CNS). The cell types include neurons, oligodendrocytes, and astrocytes. Infact, the neurospheres transplanted into an intact mouse brain can potentially differentiate into mature neurons, forming all three cell types. The potential of neural stem cells in differentiating into multi-lineage in vitro provides new avenues for the treatment of complex neurodegenerative diseases.
Mouse neural stem cells are isolated from the adult or fetal mouse brain. It is maintained in the form of a floating neurosphere in a serum-free medium with essential growth factors. The differentiated MNSCs are marked by the presence of biomarkers such as:
- β-tubulin III
- Oligodendrocyte marker O4
- Glial Fibrillary Acidic Protein (GFAP)
Mouse Brain Microglia: Interaction with Neural Stem Cells
Central Nervous System (CNS) Homeostasis
Mouse brain microglia play a vital role in cellular homeostasis. It aids in surveillance of the neural environment, sculptures neural circuits, supports neurogenesis, and clears cellular debris (phagocytosis). It supports neural circuits by eliminating redundant synapses (helps in learning and memory).
Crosstalk in Neurogenesis
The interaction of microglia and neural cells supports bidirectional communication among neurogenic niches. This promotes adequate proliferation and differentiation of neural cells. The survival of the cells is supported by paracrine signalling (in the presence of growth factors, cytokines, etc.). This influences repair mechanisms and disease progression.
Modulation of Inflammatory and Neuroregeneration
Microglia have a role in modulating immune response by influencing inflammatory markers and contribute to neuroregeneration.
Microgillia releases anti-inflammatory markers (M2-like) (IL-4, TGF-β, IL-10); and neurotrophic factors (BDNF, IGF1)
The detailed study on mouse brain microglia helps in understanding the precise role in cellular and molecular signalling in the human brain [1].
Mouse Neural Stem Cells as Neurodegenerative Disease Models
Mouse neural stem cells are a potential model for understanding neurodegenerative disease. Some critical aspects include:
Disease Model for Neurodegenerative Disease
Alzheimer’s, Dementia, Parkinson’s or spinal cord injury are all forms of neurodegenerative disease. Studies using human neural stem cells in the hippocampi of aged immunocompetent mice showed improved survival of cells, significant improvement in memory function, increased hippocampal neuronal density, and immunomodulatory properties [2].
Synaptic Repair and Neuronal Replacement Mechanisms
Promotes restoration of neural cells by secreting a broad range of neurotrophic and synaptogenic factors (BDNF, NGF, GDNF). The release of these factors enhances synaptic plasticity as well as restores synaptic density. They modulate the local inflammatory microenvironment by facilitating interaction between microglia and astrocytes.
Mouse neural cells have shown the ability to differentiate into mature neurons and glial cells, allowing neuronal replacement. The newly formed neurons can extend axons, form functional synapses and facilitate signal transmission [2].
Key Preclinical Findings
Critical evidence has come up in mouse neural stem cell research. This includes:
- Significant improvement in memory, learning, and motor function across several neurodegenerative diseases
- Neuroprotection promotion with the release of neurotrophic factors like BDNF, GDNF, and NGF
- In case of stroke or spinal cord injury, mouse neural stem cells have shown the ability to differentiate into astrocytes, neurons and oligodendrocytes and integrate into host neural circuits.
- Ability to modulate neuroinflammation
- Promotes endogenous repair by activating neural progenitor cells
- The preclinical findings confirmed the safety profile. The transplantation was well-tolerated with minimal tumorigenicity incidences [1]
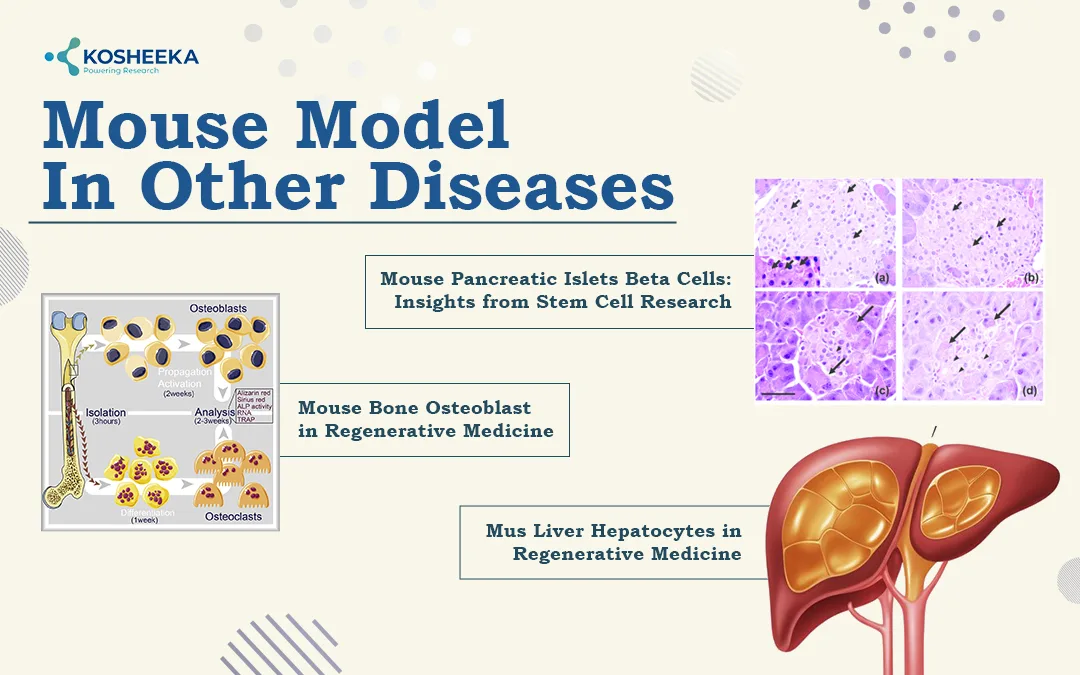
Mouse Model in Other Diseases
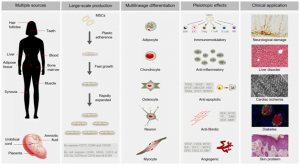
It plays an exceptional role in the in-depth understanding of various cellular pathways involved in disease. Mouse stem cells enable the development of regenerative treatment. Some other diseases widely researched include:
Mouse Pancreatic Islets Beta Cells: Insights from Stem Cell Research
Mouse Pancreatic Islets Beta Cells provide crucial insights into cell regeneration mechanisms. The crucial findings include:
- Activation of endogenous beta-cell repair
- Beta cell proliferation, survival and functional recovery
- Facilitating paracrine pathways
- Neural and MSC-derived factors showed enhanced insulin secretion
- Improves glucose homeostasis, reduces inflammatory stress
Mouse Bone Osteoblast in Regenerative Medicine
- Mouse bone osteoblasts are key in osteoblast regeneration and bone remodeling
- Regulation of Wnt/β-catenin, Notch, BMP, and Hedgehog signaling
- Governs osteoblast differentiation, matrix deposition and mineralization
- Paracrine signalling enhances osteoblast activity, improves bone microarchitecture and angiogenesis
- Therapeutic effects driven by the secretion of growth factors and immunomodulatory effects
Mus Liver Hepatocytes in Regenerative Medicine
- Mouse liver hepatocytes have proved to be a strong model in understanding liver disease
- Hepatocyte regeneration is derived from resident hepatocyte proliferation
- Paracrine signalling drives from stem and progenitor cells
- Regulates inflammation, angiogenesis, and extracellular remodeling
- Stem cell administration is helpful for liver cirrhosis, fibrosis or any other liver injury
Translational Value of Mouse Neural Stem Cell Research
Advantages
- Genetic similarity
- Well-characterized neuroanatomy
- Available in the form of a transgenic or a particular disease-specific model
Limitations
- Difference in brain size
- Immune responses
- Lifespan
- Disease progression
Relevance to Regenerative Therapies for Humans
- Provide proof-of-concept evidence for a specific study
- Enable determining optimal cell source, dosing, determining delivery route, and a feasible time for intervention
- Provide mechanistic insights on paracrine-mediated repair mechanisms, immunomodulation or synaptic regeneration
- Helps develop strategies for early-phase human clinical trials
Ethical or Regulatory Consideration
- Minimal ethical concerns
- Ethical concerns adhere to the 3Rs principle, i.e., Replacement, Reduction and Refinement
Mouse stem cells have a crucial role in regenerative research for every disease. It is essential to use high-quality stem cells for translational research.
Kosheeka, Noida, is among the leading and renowned laboratories that maintain high-quality stem cells. Stem cells for researchers and scientists can be procured for research purposes.
Future Directions in Mouse Neural Stem Cell Research
- Advanced gene editing approaches (CRISPR/Cas9)
- Development of 3D neural cultures and organoid models
- Shift to cell-free therapies.
- Biomaterials and scaffolds to target repair
- Emerging therapeutic targets
Product-Related Queries, Or Partnership Inquiries
Conclusion
The Role of mouse neural stem cells in shaping the future of regenerative medicine. The mouse model plays a crucial role in biomedical research. Mice shares anatomical and physiological similarities with humans. Their short lifespan allows a wide range of study It helps in designing preclinical human trials strategies.
References
- de Almeida, M. M., Goodkey, K., & Voronova, A. (2023). Regulation of microglia function by neural stem cells. Frontiers in Cellular Neuroscience, 17, 1130205.
- Ager R, Blurton‐Jones M, Davis J, Agazaryan A, LaFerla F. P2‐391: Human neural stem cell transplantation results in memory improvement in a 3xTg Alzheimer’s disease model mice. Alzheimer’s & Dementia. 2012 Jul;8(4S_Part_11):P399-400.
- Vlassis I, Gallicchio VS. Alzheimer’s Disease: Neural Stem Cell Therapy using 3x-Tg Mice. J Stem Cell Res. 2021;2(1):1-1.
FAQ’s
Q- What are mouse stem cells?
Mouse stem cells are referred to as undifferentiated cells that are derived from mice. They possess the ability to self-renew and differentiate into specialized stem cells. These cells are widely used for understanding disease mechanisms, drug effects and regenerative therapies.
Q- Why are mouse models used in stem cell research?
Mouse models shared high genetic and physiological similarities with humans. They are easy to manipulate genetically. These cells are considered well-established in disease models and are ideal candidates for preclinical research.
Q- How do mouse neural stem cells contribute to regenerative medicine?
Mouse neural stem cells aid in restoring function through synaptic repair, immune modulation, neuroprotection, neuronal replacement, etc. Such findings provide crucial insights for therapies for humans.
Q- Is mouse neural stem cell therapy safe?
In preclinical studies, mouse stem cell transplantation is generally safe. It is essential to use high-quality stem cells for research purposes. For human use, long-term clinical studies are recommended.