Human skin acts as the first line of the body’s defense, that constantly encounters mechanical damage, environmental stress, or exposure to chemicals. Human dermal fibroblasts are metabolically active, dynamic cellular networks found underneath the epidermis. They mainly promote:
- The production of extracellular matrix (ECM),
- Preservation of structural integrity, and
- Wound healing.
Accordingly, with increasing interest in regenerative medicine, tissue engineering, drug screening, and cosmetic science, these fibroblasts have emerged as key tools in research laboratories worldwide.
Biological Importance of Human Dermal Fibroblasts
Human Dermal Fibroblasts distribute uniformly in the dermis and spatially in a dispersed manner. Moreover, these subpopulations are morphologically and functionally specific, and different in terms of gene expression patterns:
- The reticular fibroblasts form the dense collagen network that makes the skin form tensile strength.
- Similarly papillary fibroblasts found below epidermis promote keratinocyte proliferation and basement membrane homeostasis.
Together, these subsets of Human Skin Fibroblast Cells contribute to ECM turnover, cytokine secretion, wound contraction, and angiogenic signaling.
Fibroblasts are also key responders to environmental cues. For instance, introduction of phenotypic changes due to senescence, altered collagen synthesis and excessive matrix metalloproteinases production can occur by exposure to UV radiation, inflammatory cytokines, and oxidative stress. Such molecular processes are the basis of skin aging and poor wound healing, two domains in which fibroblast cell culture models can offer valuable translationally relevant information.
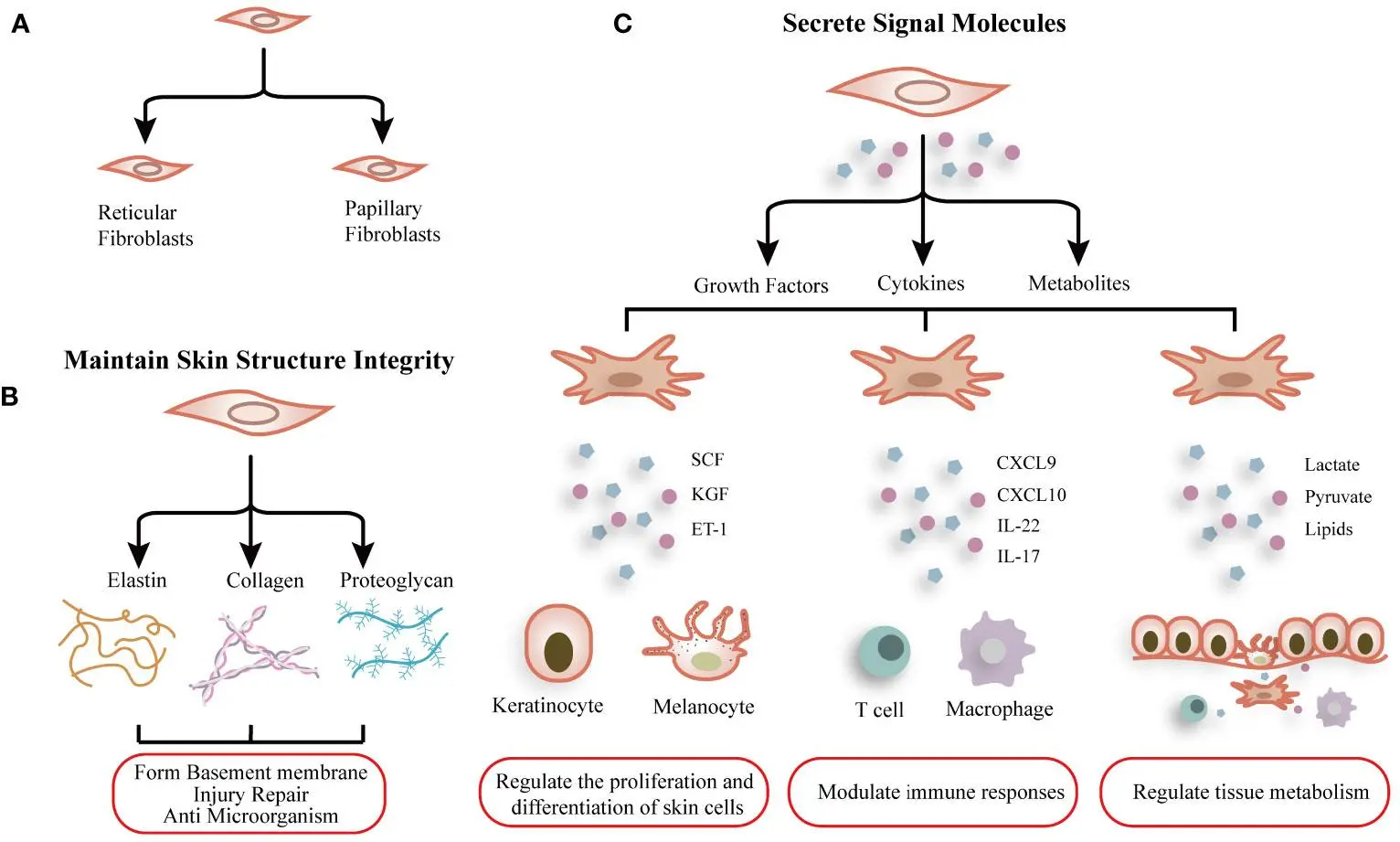
Schematic graph showing the function and heterogeneity of dermal fibroblasts. (Shi Z, Liu Z, Wei Y, Zhang R, Deng Y and Li D (2024). Front. Immunol. 15:1379490; CC BY 4.0)
Human Fibroblast Cell Lines as Experimental Platforms
The availability of standardized Human Fibroblast cell lines has significantly expanded the scope and reproducibility of skin research. Unlike primary fibroblasts that rely on donors and have limited passage number, stable fibroblast cell lines have predictable growth properties and genotype, hence compatible with high-throughput assays.
Commonly employed human fibroblast cell lines (including HDFa, neonatal foreskin-derived lines, and BJ fibroblasts) show distinct ECM production patterns and growth kinetics. They are work well for:
- Drugs and cytotoxicity screening, and
- Mechanistic investigations of cell signaling, fibrosis, and extracellular matrix remodeling owing to their stable phenotype and compatibility with a broad range of culture setups.
These cell lines are also of significance in biomanufacturing studies.
Advances in gene-editing technologies, including CRISPR/Cas9, have enabled the creation of fibroblast lines expressing reporter constructs or disease-specific mutations, which serve as robust models for studying monogenic skin disorders, collagenopathies, and mechanisms of fibroblast senescence.
Dermal Fibroblast Culture: Techniques and Applications
A culture of dermal fibroblasts is a standardized yet flexible method that facilitates the manipulation of fibroblast physiology under defined conditions.
Primary cultures are often prepared using surgical skin biopsies, neonatal foreskin or adult abdominal and facial skin. Tissue explants are usually enzymatically digested by collagenase type I or dispase to obtain viable fibroblasts.
Cultures of dermal fibroblasts need to be initiated in nutrient-rich media supplemented with fetal bovine serum, growth factors, and physiological oxygen levels to allow optimal growth.
Other parameters of culturing, such as stiffness of substrate, serum concentration, and passage number, can have a great impact on the phenotype of fibroblasts. For instance:
- First, soft hydrogels mimic compliant tissues such as papillary dermis and promote a regenerative fibroblast phenotype,
- Second, stiffer matrices encourage a more contractile, reticular-type fibroblast profile, and
- Third, high-passage cultures may exhibit senescence-associated secretory phenotypes, which are increasingly being used in studies on aging.
Advanced culture systems—including 3D collagen matrices, microfluidic chips, and co-culture platforms with keratinocytes—recapitulate the interactions between epidermal and dermal compartments. These biomimetic systems, therefore, allow detailed evaluation of fibroblast-driven epidermal regeneration, basement membrane assembly, and inflammatory signaling.
Regenerative Medicine and Wound Healing Applications
Human Dermal Fibroblasts are regenerative, thus making them the center of therapeutic research. In turn, fibroblast skin substitutes, engineered dermal matrices, and cell-seeded scaffolds are now under consideration in the management of chronic wounds, repair of burns and reconstructive surgery.
Conditioned media, abundant in cytokines, exosomes, and ECM proteins, which are produced by fibroblasts, have been found to improve keratinocyte proliferation and wound healing. Therefore, these secretomes are of specific interest for the development of cell-free therapies, which help avoid the regulatory hurdles associated with transplanted cells.
Moreover, autologous fibroblast transplantation has demonstrated clinical benefits in treating acne scars, photoaging, and atrophic dermal defects. Consequently, the inherent biocompatibility, low immunogenicity, and relative ease of expansion make fibroblasts attractive candidates for personalized regenerative applications.
Role in Anti-Aging, Toxicology, and Drug Screening
Human skin fibroblast cells are the most common, important determinants of dermal architecture that find extensive application in cosmetic sciences, toxicology, and pharmacological studies. For instance, assays based on fibroblasts give quantitative analysis of:
- collagen and elastin synthesis
- matrix metalloproteinase activity
- oxidative stress response
- cellular senescence markers
- mitochondrial health, and
- cytotoxicity and irritancy of topical compounds
These evaluations enable the identification of bioactive molecules, peptides, antioxidants, and small molecules capable of preserving dermal integrity or reversing age-associated ECM degradation.
Modeling Skin Disorders and Fibrotic Diseases
Human Fibroblast Cell Lines and primary cultures are utilized extensively to model pathological conditions such as:
- Hypertrophic scarring Keloids,
- Systemic sclerosis and dermal fibrosis,
- Ehlers–Danlos syndrome,
- Chronic non-healing wounds, and
- UV-induced photodamage.
In addition, mechanistic elucidation of TGF-2 signaling, epithelial-mesenchymal transition (EMT), collagen cross-linking defects, and inflammatory cascades can be performed using fibroblast-based models. Accordingly, these lessons can be used to design anti-fibrotic therapies and biologics against ECM dysregulation.
Conclusion
In conclusion, human dermal fibroblasts, be it primary cultures or established human fibroblast cell lines, remain crucial in biomedical studies. Moreover, regulated fibroblast culture models offer an important understanding of wound healing, ECM biology, ageing, and skin pathologies. Their application will continue to lead regenerative medicine and therapeutic development with some of the latest advancements like 3D systems, genomics, and single-cell profiling.
Product-Related Queries, Or Partnership Inquiries
FAQ’s
Q- How are human dermal fibroblasts usually cultivated in a lab setting?
These cells are typically grown in DMEM supplemented with glutamine, antibiotics, and fetal bovine serum. Additionally, to preserve viability, phenotypic stability, and replicative capacity for experimental use, they need sterile conditions, frequent medium changes, and sub-culturing prior to reaching confluence.
Q- Why are human dermal fibroblasts useful for regenerative medicine?
Human dermal fibroblasts aid in the production of extracellular matrix, tissue remodeling, and healing of wounds. They are valuable for skin biology studies and developing therapeutic and regenerative approaches, as they are significantly responsive in advanced platforms (such as 3D cultures and microfluidic systems), grow robustly, and are biocompatible.
Q- How does the dermal fibroblast help in regenerative research?
Dermal fibroblast is a model for various cellular processes, including wound healing, understanding ageing, scarring and chronic wounds and their healing. The use of dermal fibroblasts in regenerative research enables researchers to focus on these problems and develop regenerative solutions in the laboratory environment, which can further transform into therapeutic applications.
Q- Why is the dermal fibroblast a popular choice in translational research?
Dermal fibroblasts are easy to culture; they are highly responsive to stimuli and efficiently mimic the behaviour of human skin. Hence, for researchers, it acts as an ideal candidate for drug testing, the development of new biomaterials, and therapeutic studies.